Dear New World Investor:
Last Friday’s July payrolls report showed only 73,000 new nonfarm jobs added, much less than the 104,000 expected. That was the lowest number in 10 months and much less than June’s originally reported 147,000 jobs.

As you know, I think the monthly numbers are mostly statistical garbage. June was revised down from 147,000 to just 14,000, while May was revised down from 144,000 to 19,000. That tells you what’s really going on – the economic models are way behind the real economy, which is tuning down. The -253,000 two-month downward revision to payroll employment is the largest since 1979, other than the Covid-related revision in April 2020, and the largest outside recessionary times since 1968.

The May revision was mostly in government and professional/business jobs, while the June revision was overwhelmingly in government, followed distantly by retail and leisure/hospitality.

So are we in a recession? Payrolls are one of the “Big 6” indicators the National Bureau of Economic Research, the official arbiter of these things, tracks to determine whether the US has entered a recession. I don’t think we’re quite there yet, but it’s a close call.

@JimPaulsen pointed out that if you average the economic reports for the first and second quarter to smooth out the jump in tariff-anticipating imports in the March period followed by the collapse in imports in the June period, real GDP grew at an annualized rate of only 1.21%, and nonfarm employment grew at only 0.65%. That’s stall speed – and the Fed knows it. At the July 30 meeting they said the economy is OK and they can afford to be patient. It’s not OK.
The neutral rate of interest is the theoretical short-term real interest rate that maintains the economy at full employment and stable inflation. It is the rate at which monetary policy is neither stimulative nor restrictive. The current real Fed funds rate has been hovering about 1% above the neutral rate during the last couple of years, which is higher than about 90% of the time since 1961. They’re going to cut.

According to the CME FedWatch Tool,the probability of a Fed interest rate cut at the September 17 meeting has surged to 91%, up from just 38% a week ago. Unless we get an inflation shock, I think a cut is a sure thing.
Market Outlook
The S&P 500 dropped and then fought its way back to dead flat since last Thursday, so it is still up 7.8% year-to-date. The Nasdaq Composite did a little better and gained 0.6%. It is up 10.0% for the year. The SPDR S&P Biotech Exchange-Traded Fund (XBI) was up all week until it dropped today, falling 0.4% for the week. It is down 5.3% year-to-date. The small-cap Russell 2000 “soared” 0.1% – at least it was up – but is down 0.7% in 2025.
The fractal dimension did a little reversal of last week’s big down candle, as if to say: “Yeah, well, I guess the uptrend is still on. Sorta.” I’d like to see another big earnings-driven up candle next week to lock this in, because lately there’s been a downdraft after Labor Day as the wise guys try to pick up some pennies.
Top 5
Changes this week: None
Near-Term – chronological order
AKBA Akebia Therapeutics – Vafseo launch
SCYX ScyNexis – Resolution of GSK situation
EQT EQT – natural gas price rebound
USL United States 12 Month Oil Fund, LP – crude should rise quickly
FCX Freeport McMoRan – copper shortage
Long-Term – alphabetical order
ABCL AbCelllera – Will become a huge pharma royalty company
UUUU Energy Focus – Domestic uranium supplier
EQT EQT – largest US natural gas company
IBIT iShares Bitcoin Trust – Bitcoin is headed for $150,000
META Meta – a (the?) leader in the metaverse
PLTR Palantir – a (the?) leader in AI applications software
SCYX ScyNexis –First new antifungal in 20 years
Economy
The Atlanta Fed’s GDPNow model early outlook for September quarter real GDP is, once again, for an upside surprise: +2.5% versus the Blue Chip economists at +0.7%. It’s early days, though.
Coming Events
All times below are ET, and most presentations and slides are archived on the companies’ websites so you can listen to them.
Friday, August 8
SAND – Sandstorm – 11:30am – Earnings conference call
Monday, August 11
MU – Micron – 11:00am – KeyBanc Technology Leadership Forum
ON – Onsemi – 12:00pm – KeyBanc Technology Leadership Forum
Tuesday, August 12
Consumer Price Index – 8:30am
CMPS – Compass Pathways – 4:00pm – Canaccord Genuity Growth Conference
INO – Inovio – 4:30pm – Earnings conference call
FSLY – Fastly – 5:30pm – KeyBanc Technology Leadership Forum
QUIK – QuickLogic – 5:30pm – Earnings conference call
Wednesday, August 13
AKBA – Akebia Therapeutics – 1:30pm – Canaccord Genuity Growth Conference
Big Tech: The Biotech & Digital Dominators MegaShift
There are at least four ways to make money in the stocks of these large, growing, dominant companies. You can:
* * Buy a stock and hold it
* * Buy a stock and write a call option against it
* * With a Level IV options account, write an out-of-the-money put option
* * With a Level IV options account, write an out-of-the-money put option and use part of the premium to buy an out-of-the-money call option
Apple (AAPL – $220.03) announced plans to invest an additional $100 billion in US manufacturing commitments, in addition to their prior $500 billion investment in US spending, which includes working with partners to build an AI server plant in Texas. This is a response to the Trump Administration’s pressure to manufacture the iPhone in the US, which included a threat to impose a 25% tariff on iPhones if the company didn’t comply.
The announcement comes as Apple got a waiver from both the new “don’t buy Russian oil” 25% tariff on goods destined for the US from India and the existing 25% levy Trump previously said he would apply to the country’s products. It sure is nice to have friends in high places! Apple builds the majority of the iPhones headed to the US in India, after diversifying its supply chain beyond China following the pandemic. They still produce iPhones for the rest of the world in China. AAPL is a Buy under $205.
Gilead Sciences (GILD – $110.28) reported a double beat, with June quarter revenues up 1.9% from last year to $7.08 billion, above the $6.96 billion consensus estimate. Pro forma earnings per share of $2.01 were above the $1.96 consensus. HIV and oncology revenues were up:

But other product areas fell year-over-year:

On the conference call (AUDIO HERE and SLIDES HERE), CEO Daniel O’Day said: “Our strong growth this quarter was driven by Biktarvy, Descovy, Trodelvy, and Livdelzi, reflecting the diversity of our portfolio. As we enter the third quarter, we are increasing revenue and earnings guidance for the year, and look forward to delivering continued innovation and growth across our core therapeutic areas.”
He raised their 2025 net product sales outlook $100 million from $28.20-$28.60 billion to $28.30-$28.70 billion versus the consensus of $28.73 billion. He also raised earning guidance from $7.70-$8.10 to $7.95-$8.25 per share. The consensus was at $8.00.
Gilead has a robust pipeline with numerous upcoming catalysts to drive the stock higher. And you get a 3% dividend while you wait!
During the quarter, Gilead generated $827 million in operating cash flow. Even after paying $994 million in dividends and buying back $527 million of stock at the end of June, they had $7.1 billion of cash. GILD is a Long-Term Buy under $115 for a first target of $150.
Onsemi (ON – $47.59) reported June quarter results Monday before the open, with revenues down 15.5% to $1.47 billion, just above the $1.45 billion consensus estimate, and pro forma earnings of 53¢ per share, right on the 53¢ consensus.
On the conference call (AUDIO HERE and SLIDES HERE and TRANSCRIPT HERE), CEO Hassane El-Khoury said: “We are beginning to see signs of stabilization across our end markets, and we remain well-positioned to benefit from a market recovery. As we execute near-term priorities, we are positioning the company for long-term growth through investments in next-generation technologies to accelerate our market leadership.”
He guided the September quarter to revenues of $1.465 billion to $1.565 billion with pro forma earnings of 54¢ to 64¢ a share. Both were a skotch above Wall Street’s $1.50 billion and 58¢. Yet the stock tanked about 15% on Monday. Why?
Well, BofA downgraded the stock from Buy to Neutral and cut their target price from $70 to $56. They cited a muted sales recovery, including a miss in industrial sales and ongoing exits from non-core segments; continued weakness in U.S./European auto; increasing reliance on China EV, which is to be expected, but often harder to track and sustain; and limited/no gross margin recovery for several quarters. They think 2026 sales growth is likely to be closer to about 5% year-over-year versus the consensus for 10%, given the nearly $300 million headwind from non-core exits. They wrote: “We still believe ON offers the most EPS leverage in the industry and is executing on an interesting data center opportunity, but the recovery timing and data center growth isn’t likely to shine until the second half of next year, leaving the stock potentially range-bound in our view.”
Automotive had a 4% revenue decline from the March quarter driven by weakness in America and Europe, but ON expects growth in the September period with the continued EV ramps and strong traction in China, where revenue grew 23% sequentially. They announced that select Xiaomi YU7 electric SUV models will feature an advanced 800V drive platform powered by Onsemi’s EliteSiC M3e technology. It delivers industry-leading efficiency, power density, and thermal performance, enabling the development of electric vehicles with longer range, faster acceleration, and greater reliability.
June quarter AI data center revenue nearly doubled again from last year, driven by strategic partnerships and their intelligent power semiconductors. Hassane outlined further transformation through end-of-life of legacy products (approximately 5% of 2025 revenue will not repeat in 2026 due to legacy exits), and exiting non-core businesses. ON is repositioning their image sensing portfolio toward Advanced Driver-Assistance Systems and machine vision.
CFO Thad Trent said the second quarter began to show the benefits of the recent structural changes, with a substantial reduction in operating expenses. They increased their 2025 stock buyback target to 100% of free cash flow. Their 2027 targets are achievable:
On balance, I think this quarter showed stabilization in their core markets and early signs of recovery, particularly in automotive and AI data center. They are moving ahead with portfolio rationalization, operational efficiency, and next-generation technology investments. The strategic exits and repositioning will result in a higher-margin, more resilient business better positioned for long-term growth. The drop in the stock creates an excellent entry point. ON is a Buy under $60 for a $100 first target.
Palantir (PLTR – $182.20) reported an excellent June quarter double beat with revenues up 47.5% to their first-ever $1.0 billion quarter, ahead of the $939.47 billion consensus estimate, and pro forma earnings of 16¢ per share, also ahead of the 14¢ consensus. During the quarter, they closed $2.27 billion of total contract value, up 140% from last year. That included 157 deals worth $1 million or more, of which 42 deals were over $10 million. Their total customer count increased 43% to 849. Their top 20 customers now average $75 million a year in trailing 12-month revenue, up 30% from a year ago. Revenue was boosted by a 92.5% year-over-year gain in US commercial sales to $306 million, beating the estimate for $273 million, and a 53.2% rise in government contracts to $426 million, beating the estimate for $391 million.

On the conference call (YOUUBE HERE and SLIDES HERE and LETTER TO SHAREHOLDERS HERE and TRANSCRIPT HERE), CEO Alex Karp said: “The growth rate of our business has accelerated radically, yet we see no reason to pause, to relent, here…It has been a steep and upward climb – an ascent that is a reflection of the remarkable confluence of the arrival of language models, the chips necessary to power them, and our software infrastructure, one that allows organizations to tether the power of artificial intelligence to objects and relationships in the real world.”
“For a startup, even one only a thousandth of our size, this growth rate would be striking, the talk of the town. For a business of our scale, however, it is, we continue to believe, nearly without precedent or comparison.”
He guided September quarter revenue to $1.083-$1.087 billion, above the $981.999 million consensus. That will be 50% year-over-year growth and the highest sequential quarterly dollar revenue growth in Palantir’s history. He raised full year 2025 revenue guidance to $4.142 billion-$4.150 billion, above the $3.90 billion consensus. That includes US commercial revenue guidance to in excess of $1.302 billion, a growth rate of at least 85%. He raised adjusted free cash flow guidance to between $1.8 billion and $2.0 billion.
Alex said: “..our primary sales force now, and I think likely in the future, are going to be current customers telling other customers.” Customer leadership is now seeking enterprise-wide rollouts from the onset.
Wall Street’s reaction was very positive. Before the earnings report, RBC Capital Markets wrote: “We cannot rationalize why Palantir is the most expensive name in our software coverage. Absent a substantial beat-and-raise quarter elevating the [near-term] growth trajectory, valuation seems unsustainable.” Palantir then delivered beat-and-raise results.
Wedbush maintained its Outperform rating and raised their target price from $160 to $200. They wrote: “Palantir announced its FY2Q25 results featuring another quarter of strong beats across the board while raising its FY25 guidance again as the company’s marquee AIP product moat gains further traction across enterprise and federal landscapes with the AI demand wave not slowing down. We believe in the next few years Palantir has the potential to be a trillion dollar market cap as the AI Revolution takes hold We believe Palantir has a golden path to become the next Oracle over the coming years and will grow into its valuation.”
Morgan Stanley kept its Equal-Weight rating on PLTR but raised their target price from $98(!) to $155. They pointed out that the June quarter marked eight straight quarters of revenue growth acceleration coupled with significant margin expansion. Strength was driven by U.S. commercial, which accelerated again to +93% from +71% in the first quarter, and government, which accelerated to +49% from +45%.
Piper Sandler kept their Overweight rating and raised their target price from $170 to $182. They noted that the US Army awarded a contract transitioning all 75 existing contracts in which Palantir is currently a prime or subcontractor to a firm fixed-price, ten year, $10 billion enterprise agreement. Although this doesn’t increase any immediate revenues, they think it strengthens the case for further government share gains within the large $1+ trillion Defense total addressable market (TAM). They wrote: “While PLTR carries a rich valuation premium and remains a high-risk investment, the one-of-a-kind growth+margin model puts it into a unique category of one that warrants a premium, in our view.”
Cantor reiterated their Neutral rating while increasing their target price from $110 to $155. They pointed out that management’s guidance for the US Commercial business implies sustained nearly 90% growth despite continued strong comparisons in the second half of 2024. They wrote: “Palantir remains a leader in leveraging AI secular growth trends, which appear to be accelerating industry-wide and for Palantir, with our Neutral rating informed by valuation continuing to discount this industry-leading position.”
It’s hard to argue with that. Palantir is the leading AI software company and AI is eating the universe. We bought this one right at $21.40 in May, 2024. It blew through my $150 target, then rose $12.61 or 7.85% on Tuesday. It’s very tempting to take the 750% profit, isn’t it? Alex described the June quarter as “once in a generation, truly anomalous,” which makes me want to keep my sneakers laced. But I’m not sure we’d be able to buy PLTR back at a substantially lower price than it closed at today, and I am sure we want to own the AI software leader for the next five years as it “grows into its valuation.” So I am keeping PLTR as a Hold and will put it back on the buy side if there’s any meaningful weakness.
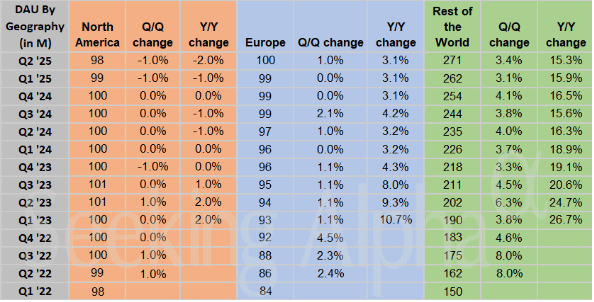
Snap (SNAP – $7.54) reported June quarter revenues up 8.9% from last year to $1.35 billion, in line with estimates but not – gasp! – a beat. The GAAP earnings per share loss of 16¢ was – gasp! – a penny worse than the minus 15¢ estimate. The Snapchat community grew to 932 million global monthly active users (MAU), an increase of 64 million or 7% year-over-year. Daily active users (DAU) grew to 469 million, an increase of 37 million or 8.6% year-over-year, but that was the slowest percentage growth in several years.
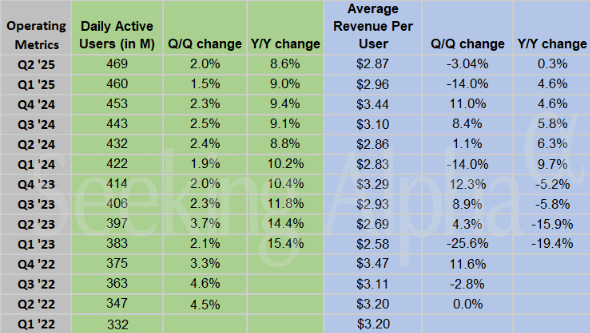
Average revenue per user (ARPU) was $2.87 in the quarter, below the consensus estimate of $2.89. ARPU only showed strength in Europe.
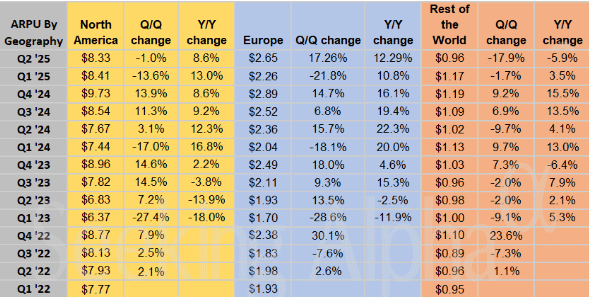
And they actually lost Daily Average Users in North America for the second quarter in a row. That’s not good.
On the conference call (AUDIO HERE and SLIDES HERE and INVESTOR LETTER HERE and TRANSCRIPT HERE), CEO Evan Spiegel said: “Our rate of topline growth was impacted by a number of factors in Q2, including an issue related to our ad platform, the timing of Ramadan, and the effects of the de minimis changes. Unfortunately, in our efforts to improve advertiser performance, we shipped a change that caused some campaigns to clear the auction at substantially reduced prices. We have since reverted this change and advertising revenue growth has improved as advertisers adjust their bid strategies to achieve their objectives.”
As a result, second quarter advertising revenue grew at its slowest pace in more than a year, rising roughly 4% to $1.17 billion, $50 million less – gasp! – than the $1.22 billion expected by Wall Street.
Evan guided September quarter revenues to $1.48 billion to $1.51 billion, a smidge above the $1.48 billion estimate. He guided daily active users to ~476.0 million, a smidge below the 476.4 million estimate.
The company will have a transformative introduction of the 6th-generation Specs next year. Evan said: “We have made a long term and consistent investment in augmented reality, committing more than $3 billion over the past 11 years to develop the world’s only full stack, vertically integrated augmented reality platform. With one of the world’s largest AR developer communities, purpose-built developer tools, a proprietary rendering engine, our own highly optimized operating system, our own optical engine, as well as the design of the hardware itself, our tight control over each aspect of the hardware and software allow us to deliver a product experience that is unmatched. We’re excited about our progress as we work to make Specs available to the public in 2026…
“In Q2 we announced plans to publicly launch our first fully standalone lightweight Specs AR glasses in 2026, marking an exciting milestone for our company and a critical step toward realizing our long-term vision for augmented reality. Snap is uniquely positioned as the only company in the world with a fully-integrated AR computing stack.
“Our upcoming Specs represent a leap forward in human-centered computing. They will be significantly smaller, lighter, and more capable than our 5th generation Spectacles released to developers in 2024. By combining advanced machine learning and AI with spatial intelligence, Specs will enable users to interact with computing in fundamentally new ways, delivering digital experiences embedded directly into the world around us.”
Wall Street really didn’t like the slow growth and took the stock down $1.61 or 17.1% on Wednesday. RBC Capital Markets wrote: “SNAP had a tough Q2. Most importantly, Q2’s execution on ad platform development & surface expansion efforts did not go according to plan, which will continue to reinforce the bear case that SNAP cannot break out of being a smaller ad platform lacking the ability to durably grow its direct response business in-line with the market.”
The real issue with Snap is whether Evan Spiegel is capable of following Mark Zuckerberg’s lead to create an AR-based, advertising-funded juggernaut. I think he can, so take advantage of this dip. The company ended the quarter with $2.9 billion in cash. SNAP is a Buy under $11 for a $17+ target.
SoftBank (SFTBY – $43.33) reported June quarter revenues up 7.1% from last year to $12.35 billion with GAAP earnings of $2.86 billion or $1.98 per share.
On the conference call (FULL VIDEO HERE and SHORT VIDEO HERE and SLIDES HERE and TRANSCRIPT HERE), CFO Yoshimitsu Goto said the better-than-expected results show their AI investments are paying off. They plan to invest an additional $22.5 billion into OpenAI by the end of the year to complete the remaining $30 billion investment together with co-investors. As of the end of June, SoftBank’s investment in OpenAI stood at $9.7 billion. Goto said: “It’s so wonderful that we can do AI business with OpenAI.”
But Goto said it is taking longer than expected to get the Stargate project off the ground, acknowledging for the first time that the $500 billion AI collaboration with OpenAI is slowing down. During the call he said that all preparations are taking place at the same time. “Although it may be taking a little bit more time than we originally expected, but we will make sure that we will be able to make good progress on this.”
He added that the project progress of Stargate has been going slower than expected. “That was my feeling, my personal observation. One of the reasons is site selection. Of course, there are a lot of options, and it’s been taking time to select a good site. And there are a lot of stakeholders. To build consensus, we need to have a lot of discussions and also technical issues and construction issues. There are a lot of things that we need to look at.”
The company finished the quarter with $28.4 million in cash. The stock is selling at a 50% discount to the value of its publicly-traded securities minus its corporate debt – SEE HERE – with many more to come:
That’s why Masa keeps buying back stock – he’s paying 50¢ for every $1.00 he gets. Do as Masa does: SFTBY is a Buy under $35 for a first target of $50 and then higher as the discount to hard book value disappears.
Small Tech
Enovix (ENVX – $11.00) has closed above $10.50 for the last 14 days. The earliest expiration date for the shareholder warrants remains August 19, subject to ENVX continuing to trade above $10.50 for 20 of 30 trading days. Don’t let your warrants expire worthless! I recommend exercising them to buy stock at $8.75 right now, before they begin commercial shipments, but if you already have a full position be sure to sell them. Their symbol is ENVXW. ENVX is a Buy up to $20 for a 4-year hold to $100+ as their BrakeFlow lithium-ion battery takes market share.
Primary Risk: A new competitor invents a better battery.
Fastly (FSLY – $7.46) reported June quarter revenues up 12.3% from last year to $148.70 million, above the $144.86 million consensus estimate. The pro forma loss of 3¢ per share was better than the 5¢ loss expected.
Remaining Performance Obligations (RPO) – the key metric measuring the total contracted revenue expected to be recognized in the future – increased 41% year-over-year to $315 million in the quarter.
On the conference call (AUDIO HERE and INVESTOR SUPPLEMENT HERE and TRANSCRIPT HERE), CEO Kip Compton said: “Fastly’s second quarter performance resulted in another record revenue quarter, outperforming both our revenue and operating loss guidance. We are raising our financial guidance for 2025 and now expect to generate positive free cash flow for the year. Our go-to-market transformation is delivering increased customer acquisition, expanded cross-sell opportunities, and market share growth.”
Kip guided for September quarter revenues of $149.0 million to $153.0 million, above the $147.38 million consensus. He expects breakeven on the bottom line, ±2¢ a share. The Street also expects breakeven.
For the full year he guided for $594.0 million to $602.0 million in revenues with a proforma loss of 4¢ to 10¢ per share. That is above the consensus for $590.02 million and a 10¢ loss, so the stock was up 94¢ or 14.42% today. Craig-Hallum upgraded the stock from Hold to Buy and increased their target price from $8 to $10. They wrote: “Re-accelerating topline, reduced cape, cost controls including reduced capitalized software, improving margins, and improved working capital are all combining to drive the second sequential $10M upward revision to the FY’25 FCF guide, with the company on track to produce their first year of positive FCF ever. Given last year’s negative -$36M in FCF was the company’s most profitable year yet, it is unlikely it was in anyone’s cards, certainly not ours, that Fastly would be getting to FCF positivity here in FY’25.”
Fastly’s enterprise customer count increased by 21 year-over-year to 622. Their top 10 customers accounted for 31% of revenue in the June quarter compared to 34% last year. Revenue from the top 10 customers increased 2% year-over-year compared to revenue growth of 17% year-over-year from customers outside the top ten. The last 12-month net retention rate (LTM NRR) increased to 104% in the June quarter from 100% in the March quarter.
They generated $10.9 million of positive free cash flow compared to $18.5 million of negative free cash flow in the second quarter of 2024. Although the stock was up 94¢ or 14.42% today, it’s still cheap. FSLY is a Buy under $10 for a 3- to 5-year hold to $50+.
Primary Risk:Content and applications delivery networks are a competitive area.
PagerDuty (PD – $15.59) was named a Leader and Outperformer in the 2025 GigaOm Radar for AIOps for the fourth consecutive year. The annual report positions PagerDuty AIOps in the Innovation half of the GigaOm Radar, based on the company’s focus on frequent product updates, expanding automation capabilities, and an AI-centric approach to human-in-the-loop decision making. PD is a Buy up to $30 for a 2- to 5-year hold as their digital operations management Software-As-A-Service gains market share.
Primary Risk: Digital operations management is a competitive area.
QuickLogic (QUIK – $5.77) reports June quarter results next week. The two analysts publishing on the stock expect revenues to be down 3.08% to $4.0 million with a loss of 7¢ a share. September quarter guidance should be for a big jump to $6.45 million as Samsung’s long-delayed cell phone ships and a profit of 7¢. QUIK is a Buy up to $10 for my $40 target as their earnings repeatedly surprise Wall Street.
Primary Risk: Customers’ product introductions and associated royalties are unpredictable.
I recommended sale of Redwire (RDW – $9.47) on June 12 at $19.32. The stock has been dropping ever since and tanked $4.23 or 30.88% today on a huge earnings miss. Unfortunately, problems like this are like cockroaches – if you see one, there are others you haven’t seen yet. Don’t re-buy RDW yet.
Biotech MegaShift
If you can afford it – and it would not be too big a position in your portfolio – putting $2,000 into each of these speculative biotechs might be a good way to start. Buying these out-of-favor, fallen, or forgotten companies that can get important products through the FDA at very low market capitalizations seems like a good strategy to me.
Risks
Development-stage biotechs are subject to investor sentiment swings from wildly optimistic to excessively pessimistic – mostly the latter recently. After the Primary Risk for each company, I’ve added the clinical stage of their lead product, the probable time of their first FDA approval, and the probable time of their next financing.
As always, you need to think about an appropriate position size. You could buy a full position upfront and then just hold on, or buy some upfront and leave room to add more on the inevitable financings, transient clinical trial setbacks, and the like.
AbCellera Biologics (ABCL- $4.24) reported June quarter revenues up 133.6% from last year to $17.1 million, a bit above the $6.4 million consensus estimate but, unfortunately, meaningless. The GAAP earnings per share loss of 12¢ also was much better than the 16¢ consensus loss estimate, and was equally meaningless.
What mattered was news on their development programs, and it was good. On the conference call (AUDIO HERE and SLIDES HERE and TRANSCRIPT HERE), CEO Carl Hansen said: “In the second quarter we hit two critical milestones, receiving authorization to initiate Phase 1 studies for both ABCL635 and ABCL575. I am pleased to announce today that we have successfully begun dosing the first participants in the Phase 1 study of ABCL635. This is a landmark achievement for AbCellera, one that completes our transition to a clinical-stage biotechnology company. Today we also announced that a third program, ABCL688, has advanced into IND-enabling studies.”
ABCL635 is a potential first-in-class non-hormonal, long-acting, subcutaneous treatment for moderate-to-severe vasomotor symptoms (VMS), commonly known as hot flashes associated with menopause. It is being developed as a next-generation NK3R antagonist with both an improved safety profile and a more convenient dosing regimen. It will be a highly differentiated product that is launched into a large and established market. The initial safety and efficacy data from this study is expected to be presented in mid-2026.
Their second drug in the clinic, ABCL575, is being developed for the treatment of moderate-to-severe atopic dermatitis. It is a subcutaneous OX40-ligand-targeting antibody engineered to support a dosing interval of once every 6 months. Dosing will begin this quarter.
The new program, ABCL688, is a potential antibody therapy for an undisclosed indication in autoimmunity. It is also the second program from AbCellera’s GPCR- and ion channel-platform to advance into IND- and CTA-enabling studies, following ABCL635. Assuming the IND-enabling studies go as planned, the company expects to submit an IND/CTA application in mid-2026.
In addition to the internal programs, during the quarter they reported five new partner-initiated program starts with downstreams, for a cumulative total of 102. Including the first two molecules from AbCellera-led programs, ABCL575 and ABCL635, the cumulative total of molecules that have reached the clinic is up to 18. Plus, their new manufacturing facility is on schedule to come on line at the end of 2025.
They still have a huge $753 million war chest, including $580 million in cash and $170 million in remaining government funding, enough to carry them “well beyond the next three years of increasing pipeline investments.” Buy ABCL up to $6 for a long-term hold to $30 or more.
Primary Risk: Partnered and owned drugs fail in the clinic.
Clinical stage of lead product: Partnered: Various Owned: Preclinical
Probable time of next FDA approval: 2027-2028
Probable time of next financing: 2026-2027 or never
Akebia Therapeutics (AKBA- $3.01) reported a double beat for the June quarter, with revenues up 43.1% from last year to $62.47 million, far above the $47.24 million consensus estimate. Breakeven earnings per share also were above the consensus expectation for a 2¢ loss. So why was the stock down 77.5¢ or 20.48% today?
In the March launch quarter, Vafseo revenues were $12.0 million. Because this was the launch quarter, it’s reasonable to think the monthly cadence looked something like $3 million – $4 million – $5 million. If March was around $5 million, the June quarter should have been north of $15 million. It wasn’t. Vafseo revenues were only $13.3 million, a sequential increase of only 10.3%. In the March quarter, more than 640 prescribers wrote an average of 12 prescriptions each. In the June quarter, that increased to only “more than 725 prescribers” with an average of 13 prescriptions each. That sounded to Wall Street like the launch has stalled.
The company said a few things that seemed to confirm that. More than 80% of the prescriptions were refills, suggesting that only a small percentage of dialysis patients will be switched to Vafseo. Akebia said that by the end of the September quarter, they expect Dialysis Clinics and Innovative Renal Care, the fourth and fifth largest dialysis organizations, will have operationalized protocols. That would enable Vafseo prescribing access to a total of more than 75,000 patients, an increase from about 40,000 patients at the end of the June quarter. That seems like a small available market. Finally, prescriptions rose 55%, again mostly from U.S. Renal Care, while revenues rose only 10.3%. That suggests the volume discount schedule is much more aggressive than any analyst thought.
DaVita,one of the two leading dialysis organization serving more than 200,000 patients, has begun ordering Vafseo to support a three-month operational pilot starting August 18 across more than 100 clinics, which will lead to the opportunity for broad prescribing before yearend. That would give Akebia a total available market of 275,000 patients by the end of the year. Akebia is talking to Fresenius, the other dialysis giant, and expects then to eventually start with a similar operational pilot.
On the conference call (AUDIO HERE and TRANSCRIPT HERE), CEO John Butler said they have requested a Type-C meeting with the FDA to start a Phase 3 clinical trial of Vafseo for treating anemia in late-stage CKD patients who are not on dialysis by the end of this year
Akebia started a post-marketing trial, conducted within DaVita clinics, to evaluate the efficacy and safety of three times per week dosing of Vafseo compared to standard of care erythropoiesis-stimulating agents (ESAs) in patients with anemia of CKD receiving in-center dialysis.
Today’s stock price drop reflects Wall Street’s impatience with the speed of the launch, but I believe this is more a reflection of their lack of understanding of the dialysis market than the ultimate success of the drug. John Butler and his team have a multi-pronged strategy to make Vafseo the standard of care in dialysis by the end of TDAPA on December 31, 2026. I have always thought they will succeed. They finished the quarter with $137.3 million in cash, which is enough to take them to profitability and positive cash flow. Buy AKBA up to $4 for the Vafseo launches in the EU, UK, and US. I still think Amgen and/or GSK, which launched and withdrew an inferior oral HIF-PH inhibitor, will make a bid for the company.
Primary Risk: Vafseo doesn’t sell in the US.
Clinical stage of lead product: Approved
Probable time of next approval: 2026
Probable time of next financing: Never
Inovio (INO – $1.44) reports June quarter results next week. Analysts expect a loss of 62¢ a share. September quarter guidance should be for a slightly smaller loss of 57¢ a share. I expect them to say they have begun the rolling submission of their Biologics Licensing Application for INO-3107 for recurrent respiratory papillomatosis (RRP).
Inovio raised $22.9 million from their July 3 stock offering. In the prospectus, they said: “Based on the planned use of proceeds described above, we believe that the net proceeds from this offering and our current cash and cash equivalents will be sufficient to enable us to fund our operating expenses and capital expenditure requirements into the second quarter of 2026.”
Assuming they complete the BLA filing process by yearend and are granted priority review, I expect them to do another offering early next year at a higher price. INO is a Buy under $5 for a very long-term hold.
Primary Risk: Their drugs fail in the clinic.
Clinical stage of lead product: Phase 3
Probable time of first FDA approval: Early 2026
Probable time of next financing:After FDA approval in 2026
Medicenna Therapeutics (MDNAF – $0.75) reported a June quarter loss of $4.9 million or 6¢ per share. CEO Fahar Merchant said: “We enter the second half of 2025 encouraged by positive clinical data from the Phase 1/2 ABILITY-1 trial, and remain on track to complete enrollment and report top-line data from both the monotherapy and combination arms before year-end.
“We are motivated by previously reported data, from each of the three tumor-specific cohorts, to pursue expedited regulatory pathways for MDNA11 in order to address critical unmet needs in patients who do not respond to blockbuster immunotherapies. In parallel, we are making strong progress with MDNA113, our lead candidate from the proprietary BiSKIT platform. This bi-functional anti-PD1-IL2 Superkine is engineered with integrated targeting and stealth capabilities, enabling tumor-specific activation while minimizing peripheral effects. We look forward to sharing further clinical updates at conferences and scientific events later this year.”
Medicenna ended the quarter with $20.5 million in cash, enough to carry them through mid-2026. Buy MDNAF under $3 for a first target of $20.
Primary Risk: Their drugs fail in the clinic.
Clinical stage of lead product: Entering Phase 3
Probable time of first FDA approval: 2026
Probable time of next financing: 2025
TG Therapeutics (TGTX – $27.89) reported June quarter revenues up 92.1% from last year and 16% from the March quarter to $141.15 million, but that was short of the $146.27 million consensus estimate. GAAP earnings of 17¢ per share were way short of the 28¢ consensus. The stock dropped about 18% on Monday after the earnings release.
On the conference call (AUDIO HERE and TRANSCRIPT HERE), CEO Mike Weiss said: “Now approximately 2.5 years into launch, we estimate that nearly 1 in every 3 new IV anti-CD20 patients are prescribed Briumvi.”
The Chief Commercial Officer, Adam Waldman, said: “US net sales for Briumvi in Q2 totaled approximately $139 million, ahead of our internal expectations and building on the robust growth we achieved in Q1, positioning us for a strong second half of 2025. We saw a meaningful increase in both new prescribers and new accounts, reflecting deeper penetration across academic institutions and community neurology practices.”
Mike raised the 2025 Briumvi revenue target from $560 million to $570-$575 million and the total revenue target from $575 million to $585 million. He said: “The strong uptake we’re seeing, combined with deepening physician confidence and compelling patient experiences, underscores the strength of our launch strategy and gives us confidence in reaching the updated guidance.”
They do expect seasonality in the September quarter with faster year-over-year growth in the December fourth quarter.
TG will start enrollment in a Phase 3 pivotal trial of subcutaneous Briumvi and is continuing enrollment in the Phase 3 trial of the streamlined dosing regimen of a single 600 milligram IV dose of Briumvi on Day #1. They also are continuing enrollment in the Phase 3 trial of Briumvi in myasthenia gravis and have dosed the first patient in the Phase 1 trial of azer-cel for the treatment of autoimmune diseases, beginning with progressive forms of MS.
The company finished the quarter with $270 million in cash. Hold TGTX for a target price in a buyout of $40 or more.
Primary Risk: Briumvi, the MS drug, fails to sell.
Clinical stage of lead product: Approved
Probable time of next FDA approval: NM
Probable time of next financing: Never
Inflation MegaShift
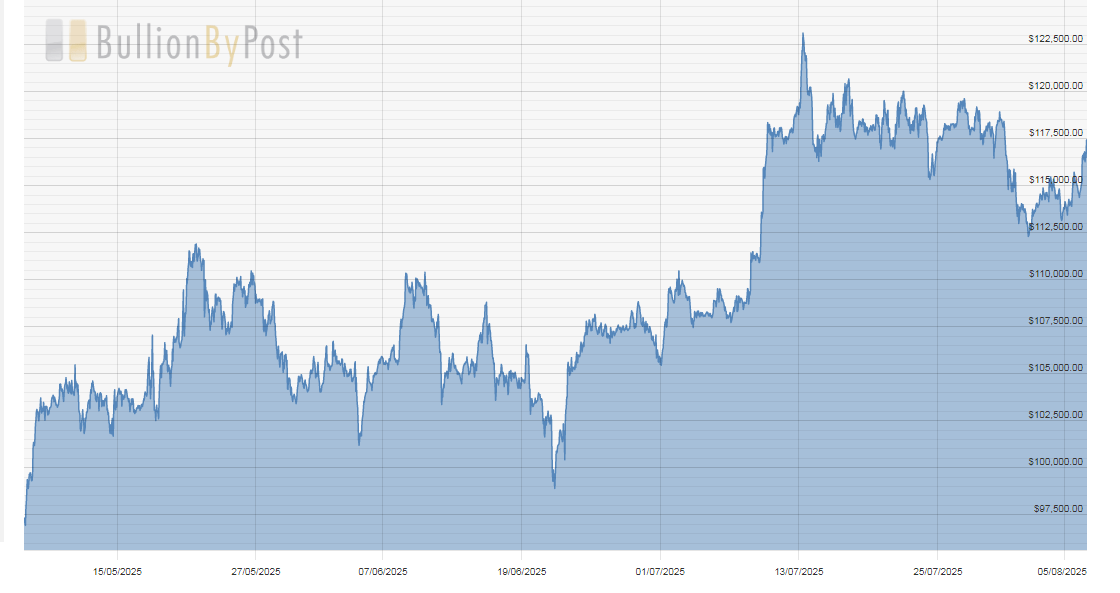
Gold ($3,472.50) hit a new high today as bets increased on a Fed rate cut and tariff turmoil continued. I said the consolidation was over and a new trend could start anytime. The fractal dimension is about to drop back through 55, signaling that new trend has started – and I’m going to need a bigger graphic. Again.
Miners & Related
Coeur Mining (CDE – $11.28) reported June quarter revenues up 116.6% from last year to $480.7 million, above the $475.3 million consensus estimate. Pro forma earnings per share of 20¢ beat the 18¢ consensus. The stock jumped $1.38 or 14.05% today
Coeur produced 108,487 ounces of gold and 4.7 million ounces of silver in the quarter. Gold sales for the quarter totaled 106,948 ounces – they held some back – at an average realized price of $3,021, compared to $2,635 in the March quarter and $2,003 last year. Nothing like a precious metals bull market!
Speaking of which, they sold all 4.7 million ounces of silver an an average realized price of $33.72 per ounce, compared to $32.05 per ounce in the March period and $26.20 per ounce last year.
On the conference call (AUDIO HERE and SLIDES HERE and TRANSCRIPT HERE), CEO Mitchell Krebs said they had $207 million in cash flow from operations and a record $146 million in free cash flow. He added: “Looking ahead to the second half of the year, we expect even higher gold and silver production levels consistent with our re-affirmed 2025 production and cost guidance. We remain uniquely positioned to leverage higher gold and silver prices, which is expected to lead to over $800 million of full-year 2025 adjusted EBITDA [Earnings Before Interest, Taxes, Depreciation & Amortization] and over $400 million of full-year 2025 free cash flow.”
Mitch said they finally are seeing inflationary cost pressures for labor, diesel, materials, and power subside. During the quarter, they repaid the remaining $110 million balance on their revolving credit facility, bought back 216,500 shares, and still finished the quarter with $112 million in cash. Their key deliverables for the second half of 2025 are:
CDE is a Buy under $10 for a $20 target as gold goes higher.
Primary Risk: Prices of precious metals fall due to US dollar strength.
Sandstorm Gold (SAND – $10.16) reported June quarter revenues up 24.2% from last year to a record $51.4 million, just under the $51.48 million consensus estimate. Cash flows from operations hit $37.7 million, supported by record cash operating margins of $2,981 per gold equivalent ounce. The conference call is tomorrow morning.
The company acknowledged and supports Royal Gold’s (RGD) acquisition of a gold stream on the Kansanshi copper-gold mine for $1 billion. Nolan said: “Sandstorm is pleased to support Royal Gold’s acquisition of the Kansanshi gold stream. Our acquisition agreement with Royal Gold specifically contemplated the potential for a large stream acquisition before closing the Royal Gold/Sandstorm transaction, and we are pleased with today’s announcement. Being part of a larger and well-capitalized entity creates the opportunity for Sandstorm shareholders to benefit from exposure to larger acquisitions. The Kansanshi transaction is an excellent example of a cash-flowing stream on a large, long-life mine with current reserves supporting a 20-year mine life from a first-tier operator. The gold stream is expected to add approximately 35,000 to 40,000 ounces per year over the next 10 years, further enhancing the combined portfolio.”
SAND is a Hold for the Royal Gold acquisition.
Primary Risk: Prices of precious metals fall due to US dollar strength.
Cryptocurrencies
Cryptocurrencies are a diversifying asset that offer a unique opportunity to make (or lose!) a lot of money quickly.
Bitcoin (BTC-USD on Yahoo – $117,413.17) rose as President Trump signed an executive order today that allows alternative assets like cryptocurrencies, real estate, and private equity into the retirement accounts of millions of Americans. The order directs the Labor Department and other agencies to redefine what would be considered a qualified asset under 401(k) retirement rules. So there will be no immediate change, because Federal bureaucrats need to rewrite rules and regulations to allow the expanded choices, and that will take months to complete. Meanwhile, Wall Street will buy crypto to front-run the 401(k) money.
BTC-USD, ETH-USD, IBIT, and ETHA are Strong Buys.
Primary Risk: Bitcoin falls due to over-regulation or is surpassed by another cryptocurrency.
iShares Bitcoin Trust (IBIT- $66.83) remains the cheapest and easiest way to buy bitcoin. IBIT is a Buy for the 2028, 2032, and 2036 halvings.
Primary Risk:Bitcoin falls due to over-regulation or is surpassed by another cryptocurrency.
Ethereum (ETH-USD on Yahoo – $3,896.40) is likely to be a huge beneficiary of the 401(k) rules change. Wall Street will fall all over themselves trying to stand out in crypto. They’ll think that any idiot can recommend bitcoin, so they’ll explain the use case for ethereum and recommend it instead. The funny part is that they’ll be right – ethereum is likely to outperform bitcoin over the next several years. I say buy both. ETH-USD is a Buy.
Primary Risk: Bitcoin extensions outperform Ethereum.
iShares Ethereum Trust (ETHA- $29.31) remains the cheapest and easiest way to buy ethereum. ETHA is a Buy for the coming explosion in token-funded start-ups.
Primary Risk: Ethereum falls due to over-regulation or is surpassed by another cryptocurrency.
Commodities
Oil – $63.82
Oil dropped from the high $60s to the low $60s. After a 16 minute meeting, OPEC+ said they will sharply hike production again in September to finish unwinding their latest tranche of supply cuts. They say they’ll add 547,000 barrels a day next month. This completes the accelerated reversal of the 2.2 million barrels a day cutback made by eight members in 2023, and also includes an extra allowance being phased in by the United Arab Emirates. The media said: “The latest hike caps a dramatic shift from the Organization of the Petroleum Exporting Countries and its partners, from defending prices to opening the taps.”
But the only tap left to open is Saudi exports, and my prediction is they are doing this to look good for President Trump with no intention of actually increasing exports. They said the latest decision was taken “in view of a steady global economic outlook and current healthy market fundamentals, as reflected in the low oil inventories.” OPEC+ decided to keep its options open regarding another layer of halted output, amounting to about 1.66 million barrels a day, which currently expires at the end of 2026.
According to the International Monetary Fund, Saudi Arabia needs oil above $90 a barrel to cover government spending. With OPEC+ in the driver’s seat as US shale production tails off, I expect MBS to get what he wants.
The July 2026 Crude Oil Futures (CLN26.NYM – $61.45) are a Buy under $70 for a $200+ target. Only buy futures for all cash; do not use margin.
The United States 12 Month Oil Fund, LP (USL – $35.46) is a Buy under $40 for a $100+ target.
Vermilion Energy (VET – $7.83) is a Buy under $11 for a target price of $24 or more.
Primary Risk: Oil prices fall.
Energy Fuels (UUUU – $9.60) reported a double miss. June quarter revenues were down 51.7% from last year to $4.21 million, less than half of the $9.40 million consensus estimate. The GAAP earnings loss of 10¢ per share was worse than the consensus for a 4¢ loss. What happened?
As often happens with Energy Fuels, they built inventory instead of selling stuff, because they expect higher pries in the future. They produced 180,000 pounds of finished U3O8 at the White Mesa Mill in Utah during the quarter, but only sold 50,000 pounds on the spot market for $77.00 per pound, realizing total gross proceeds of $3.85 million at a gross margin of 31%. As of June 30, they held 1,875,000 pounds of uranium in inventory, including 725,000 pounds of finished goods. They only expect to sell 350,000 pounds this year.
On the conference call (AUDIO HERE and TRANSCRIPT HERE), CEO Mark Chalmers said: “This quarter delivered proof that our long-term commitment to the Pinyon Plain uranium mine has been worth the effort, as the mine continues to be one of the highest, if not the highest, grade uranium mines in US history. The exceptional production at this mine is a ‘once in a lifetime event’ and has come at the perfect time for Energy Fuels, as it places us in the enviable position of increasing production while lowering costs.
“Based on the high mined grades and production so far, we anticipate sustained production and high grades at Pinyon Plain for several additional years beyond our initial estimates, which offers sustained low unit costs, possibly around $23-$30 per pound, for dramatically higher expected uranium margins. While our uranium segment showed a loss this quarter due to limited uranium sales, revenue from upcoming contract deliveries, along with possible spot market sales during the remainder of 2025, is expected to provide substantial cash flow starting this year and getting into full swing in 2026 and subsequent years, to be offset against our global operating and capital costs.”
I recommended UUUU for its uranium operations because I think the price of U3O8 will rise dramatically for several years. Their rare earth elements (REE) operation is a bonus. Rare earths are not really rare – they’re everywhere. What is rare is a low-cost supply of raw material (heavy mineral sands) and the ability to process them, which the White Mesa Mill can do.
Chinese neodymium-praseodymium prices have increased 19.5% from $61.88 to $73.93 per kilogram over the last month. Recently published European dysprosium and terbium oxide prices of $800 per kilogram and $3,625 per kilogram are over 300% higher than the published Chinese prices of $230 per kilogram and $988 per kilogram. That shows the scarcity of these REE oxides outside of China and their importance to markets in the United States and Europe. Energy Fuels is in the process of producing dysprosium oxide at pilot scale at the White Mesa Mill, with their first kilogram of dysprosium oxide expected in August, their first production of terbium oxide expected in November, and their first production of samarium oxide expected in the March quarter. I expect news releases on each successful production to drive the stock higher. They should be able to produce dysprosium, terbium, and samarium on a commercial scale as early as the December 2026 quarter.
Energy Fuels finished the quarter with $71.49 million of cash, $126.41 million of marketable securities, and no debt. UUUU is a buy under $8 for a $30 target.
Primary Risk: Uranium prices fall.
* * * * *
Yo-Yo Ma & Kathryn Stott – Ave Maria
* * * * *

So is this because neither marriage nor home ownership are goals anymore for 30-year-olds, or because they want to but can’t?
* * * * *
Your getting nervous Editor,
Michael Murphy CFA
Founding Editor
New World Investor
All Recommendations
Priced 08/07/25. Check out the complete Portfolio page HERE.
Buys
These are the stocks everyone needs to own because transformative events are happening over the next year or two, and I expect to hold them long-term.
Tech Dominators
Apple Computer (AAPL – $220.03) – Buy under $205
Gilead Sciences (GILD – $110.28) – Buy under $115, first target price $150
Meta (META – $761.83) – Buy under $705 for a long-term hold
Micron Technology (MU – $111.87) – Buy under $120, first target price $140
Onsemi (ON – $47.59) – Buy under $60, first target price $100
PayPal (PYPL – $68.22) – Buy under $75, target price $150
Snap (SNAP – $7.54) – Buy under $11, target price $17+
SoftBank (SFTBY – $43.33) – Buy under $35, target price $50+
Small Tech
Enovix (ENVX – $11.00) – Buy under $20; 4-year hold to $100+
First Trust NASDAQ Cybersecurity ETF (CIBR – $71.19) – Buy under $75; 3- to 5-year hold
Fastly (FSLY – $7.46) – Buy under $10 for a 3- to 5-year hold to $50+
PagerDuty (PD – $15.59) – Buy under $30; 2- to 5-year hold
QuickLogic (QUIK – $5.77) – Buy under $10, target price $40
ARK Venture Fund (ARKVX – $33.57) – Buy for SpaceX
$20-for-$1 Biotech
AbCellera Biologics (ABCL – $4.24) – Buy under $6, target $30+
Akebia Biotherapeutics (AKBA – $3.01) – Buy under $4, target $20
Compass Pathways (CMPS – $4.31) – Buy under $10, hold a long time for a 20x return
Editas Medicines (EDIT – $2.49) – Buy under $6 for a double in 12 months and a long-term hold to much higher prices
Inovio (INO – $1.44) – Buy under $5, hold a long time
Medicenna (MDNAF – $0.74) – Buy under $3, first target $20, then maybe $40
ScyNexis (SCYX – $0.88) – Buy under $2.50, target price $20, then $50
Inflation
A Short-Sale or REO House – ($415,400) – Hold
Bag of Junk Silver – ($38.48) – hold through silver bull market
Sprott Gold Miners ETF (SGDM – $49.43) – Buy under $50, target price $75
Sprott Junior Gold Miners ETF (SGDJ – $52.66) – Buy under $60, target price $100
Sprott Physical Gold and Silver Trust (CEF – $31.42) – Buy under $35, target price $60
Global X Silver Miners ETF (SIL – $53.83) – Buy under $60, target price $100
Coeur Mining (CDE – $11.28) – Buy under $10, target price $20
First Majestic Mining (AG – $8.73) – Buy under $11, next target price $23
Paramount Gold Nevada (PZG – $0.67) – Buy under $1, first target price $10
Cryptocurrencies
Bitcoin (BTC-USD – $117,413.17) – Buy
iShares Bitcoin Trust (IBIT – $66.83) – Buy
Ethereum (ETH-USD – $3,868.18)– Buy
iShares Ethereum Trust (ETHA- $29.31) – Buy
Commodities
Crude Oil Futures – July 2026 (CLN26.NYM – $61.45) – Buy under $70; $200+ target
United States 12 Month Oil Fund, LP (USL – $35.46) – Buy under $40; $100+ target
Vermilion Energy (VET – $7.83) – Buy under $11; $24+ target
Energy Fuels (UUUU – $9.60) – Buy under $8; $30 target
EQT (EQT – $51.24) – Buy under $70; hold for much higher prices ($100+)
Freeport McMoRan (FCX – $40.80) – Buy under $50; $70 target within two years
Holds
These are holds but not sells – yet. They could get moved back to one of the buy categories if their prices drop or outlook improves, or they could become sell recommendations in the future.
Corning (GLW – $64.76) – Hold for $70
Nvidia (NVDA – $180.77) – Hold for $180 first target price
Palantir (PLTR – $182.20) – Hold for $180 first target price
TG Therapeutics (TGTX – $27.89) – Hold for buyout at $40+
Dakota Gold (DC – $4.01) – Hold for $6 target price
Sandstorm Gold (SAND – $10.16) – Hold for Royal Gold acquisition
Publisher: GwynRose LLC. 5348 Vegas Drive, Suite 868, Las Vegas, NV 89108
New World Investor does not act as a personal investment adviser or advocate the purchase or sale of any security or investment for any specific individual. The recommendations and analysis presented to members are for the exclusive use of members. Members should be aware that investment markets have inherent risks and there can be no guarantee of future profits. Likewise, past performance does not assure future results. Recommendations are subject to change at any time. Nothing in this presentation should be considered personalized investment advice. No communication to you by Michael Murphy or any of our employees or contractors should be deemed as personalized investment advice.
Copyright ©GwynRoseLLC 2025
New World Investor Mastermind Group
1. Post unto others as you would have them post unto you.
2. Keep it clean, like a 1950s family television show. Your alter ego can run free on Twitter.
3. NO PERSONAL ATTACKS! If you don’t like the stock, don’t trash the person. Everyone is responsible for their own due diligence and investments.
4. Don’t post here about politics or religion – you aren’t going to change anyone’s mind. Again, NO PERSONAL ATTACKS!
5. The investment implications of something going on in politics or religion is OK.
6. Of course, there’s never a reason to slur someone based on race, religion, gender, sexual orientation, or country of national origin.
7. Please, no snark!
 Print This Post
Print This Post













Mm question for you,do you still feel that a bid for akba will come before the end of the summer like you recently stated, tx
Disgustingly laughable. See my post below.
SAN DIEGO, Aug. 08, 2025 (GLOBE NEWSWIRE) — Capricor Therapeutics (NASDAQ: CAPR), a biotechnology company developing transformative cell and exosome-based therapeutics for the treatment of rare diseases, today announced that a Type A meeting with the U.S. Food and Drug Administration (FDA) has been scheduled to discuss the regulatory path for its Biologics License Application (BLA) for Deramiocel, the Company’s lead cell therapy candidate for the treatment of cardiomyopathy associated with Duchenne muscular dystrophy (DMD).
To accommodate this meeting, the Company has rescheduled the release of its financial results for the second quarter ended June 30, 2025, to Monday, August 11, 2025, after the market close. Management will host a webcast and conference call at 4:30 p.m. ET on the same day to review the financial results and provide a corporate update.
Title:
Capricor Therapeutics Second Quarter 2025 Financial Results and Recent Corporate Update Conference Call and Webcast
Date:
Monday, August 11, 2025
Time:
4:30 p.m. ET
Conference Call Details:
Toll-Free: 1-800-717-1738
Too bad the bad boy Prasad is now back at the FDA. Hopefully he will do the common sense thing and approve Deramiocel without too much delay. Otherwise he will be lynched by Trump people.
The big criticism of big govt from either party is that running a big organization is nearly impossible. Prasad is a big leftist and a vocal critic of DJT. How could DJT’s staff let him in the FDA? Big families can’t function as well as smaller ones. A mother with 10 children uses older children to be surrogate parents. She could be great with a few kids, but not with 10.
Things are looking interesting re CAPR
Vinay Prasad must have a wretched family. In late July the HHS comment about his departure was that he “wanted to spend more time with his family”. Two weeks later he is back at the FDA. How did this happen? Was it because his family is so wretched that he could not tolerate spending moer time with them? Was it because the HHS statement was a lie? Obviously that couldn’t be the case because no one in the Trump administration would tell a lie (except about the Epstein files). Was it because he realized he was unemployable because he had let his Board certification lapse, so he wet to Trump begging on his knees to get one of his jobs back and let others take over ass Chief of Medicine and Chief of Science? Is there any other reason he has reappeared like Mike Myers in the Halloween slasher movie series after it appears the malignant entity has been vanquished once and for all?
In an environment where so many companies are reporting earnings beats , surprise earnings, and double beats if your CEO reports only he made his numbers or missed slightly, investors will brutally cut you off at the knees . Such is the case of TGTX, SNAP, UUUU, and RDW. Maybe the CEO’s need to hold back on their giddiness of look at me go mentality and instead sand bag their earnings estimates 10-20 percent . Then they have a better opportunity to beat their estimates and increase the share price. Just saying!!
AKBA
MM where in the launch cycle would you expect GSK and/or AMGEN to make a bid for the company. And what is the status of approval in the UK, EU AND the ROW
With poor management from Butler, AKBA is now up to a year behind in its potential. Any buyout is on hold for a few years. Nobody would offer more than $4 based on facts of today. Actually, Big Pharma should kick Butler in the ass with low offers so he can wake up. For any proposal to dilute and increase his salary, 100% of shareholders should vote HELL NO.
What did you think of the meeting yesterday re CARP Linda was good as usual I thot
I didn’t listen yet. There is a rumor that the type A meeting is tomorrow. Several people think that the most likely outcome of the entire process is accelerated approval (AA) with followup monitoring of LVEF. AA would make patients and parents very happy that they could get the life-extending treatment ASAP. AA would lessen the animosity towards a heartless FDA. AA would let FDA demons like Prasad satisfy their egos to over-regulate by giving them post-approval supervision. Sounds plausible to me. The risk to CAPR is that they have cash only for 4-5 quarters beyond June 30. Regular time consuming phase 3 procedures could bankrupt them, so AA is a great opportunity for them to survive while revenue from deramiocel sales comes in.
MM–please address my reservations about TGTX on the last board. Specifically, my discussion about patent risks and generics shutting out branded products from many companies. What is average time for extensions of patents, in general?
NWI subscribers should read the latest comments on Stocktwits from me (viber7), hsainu and others about AKBA.
Dodged a bullet and sold at $35.99!
I’ll say it again. Thanks for being smart. I did the same thing as you. We have to make our own decisions. I doubt MM will address my observations and questions about TGTX.
AKBA is another biotech over promise and under deliver from this newsletter – I don’t know why I keep believing
The value of this newsletter is from posters with good picks and insights. Do you think DECK is bottoming near $100? How long do you think tariff scares go on? Trump is making a valiant effort to defeat commies, but the mark of tyrants is that they never change. If this goes on for the remaining 3+ years of DJT, DECK could stay low, but eventually investors adjust to that reality, and DECK could recover to $200. It was $60 not too long ago, so even DECK has risks like bios, just not as bad.
Reminder, most of DECKs growth will come from outside the US as they are underdeveloped or non existent in most countries
So tariffs on US imports should not matter. Ex-US revenue will still be the same, with or without tariffs. Correct? What’s your estimate of the chance for the PPS to pull back to $40-60?
it would only be my opinion, what I do know is (1) DECK is priced at half the evaluation of competitors Nike, Adidas, Reebok (2)growth sneak today in HOKA, and top selling fashion boots in UGGS (3) they have significant international upside much more than competition. Repeating my theses on tariffs – they are causing more fear than reality because Dems need talking points to hurt Trump – as stated before, industry experts (as well as I see in my business on imports) is importers will eat half the tariff, sellers will eat a portion, and many companies are offsetting it by re-allocating marketing funds into COG to reduce cost increases. Understand that no retailer will raise prices if it erodes sales too much, they have elasticity models that show for each percent price raise how much sales declines – they cannot afford to suffer double digit declines – push comes to shove, everyone makes a little less profit in the chain.
The AKBA story is most definitely not over, not by a long shot. It is taking longer to take off, probably by 6 months or so, not 6 years.
Look at the latest posts on Stocktwits from me (viber7), hsainu, Holy_boy (nephrologist). SPARTAN9999 had the most hopeful comment–V uptake was slow or almost nonexistent among medium and large DO’s because they were waiting for USRC to be the guineapigs and iron out potential problems with V. Indeed, there was a computer glitch at USRC whereby if a patient didn’t respond to early use of V and needed ESA rescue, V was stopped. The proper treatment would have been to maintain or titrate V to a higher dose while adding judicious occasional ESA. This is a BIG BIG BIG problem with use of computers and AI to take over patient management. Nephrologists like Holy_boy are much better equipped to do the best management. So USRC corrected the problem in Q2. Still, USRC managed to get 35% of their eligible TDAPA patients on V in only 2 quarters–excellent launch at USRC. Now it is up to the larger DO’s to get the message and start selling V. In my latest post, I humored hsainu and accepted his eventual projection of $1 billion in annual revenue from V while TDAPA is still applicable. Trouble is, they will be lucky to get blockbuster revenue by the end of TDAPA, late 2026. In 2027, revenue drops 80% to $2500 to be competitive with ESA. I showed his error in using P/S multiples of 10 for $1 billion sales. It is appropriate to use post-TDAPA revenue of $0.2 billion starting in 2027, then P/S of 10 for a growing company yields market cap of $2 billion. 265 million outstanding shares, is PPS of $8 at that time, IF IF IF IF V shows superb market penetration. It might do that since V has advantages over ESA for ease of use, safety, comparable efficacy. I think NDD for V will be approved in 2027-8. If AKBA gets TDAPA for V used in NDD, they can get 5X revenue again, and the PPS can be 3-5X $8, or $24-40. But I view that as a TDAPA scam. How can they get $15K for V used for NDD but only $2.5K for the same V used for DD? Realistically, I would double the PPS target upon NDD approval, or $16.
For new money, buy up to $4 only with a small sum, My average cost is $1.75, but I won’t buy any more at current prices. I am already overweight in AKBA.
Are you still bullish on AKBA? It sounds like you are ticked off with the CEO’s latest antics? Thanks for all your input. Much appreciated.
This AM, I replied to big bull hsainu on ST. It is one thing to say that many more patients will be accessible in the next few quarters, but another to speculate what % of these accessible patients will actually get scripts. I think that large DO’s will follow the lead of USRC, letting the small guys like USRC iron out the problems first. The optimistic view of hsainu is possible–$1 billion in sales by 2026 end. But I will double my price target from yesterday based on the following. Magic_money reminded us, and hsainu confirms, that after 2026, TDAPA doesn’t fall off the cliff, but is gradually reduced. It will be extended for 3 years, with revenue in 2027 at 65% of full TDAPA, 2028 will be 65% X 65% = 42%, 2029 will be 65% X 65% X 65% = 27%. My PPS targets yesterday were based on 20% after TDAPA. This gives AKBA another 3 years to get uptake much higher than 25% which is required for $1 billion under TDAPA. So multiply my $8 to $16 in 2027-8 under hsainu’s optimistic scenario. If John Butler moves his ass and gets 35% uptake from the large DO’s as he got from USRC in 2 quarters, the optimistic scenario could work out. But with continued slow uptake, PPS could still be only $4-5 for the next few years. It depends on your estimate of the situation and your entry point.
Early success in bios gets many investors excited, so they remain bullish for far too long. I was caught up in TGTX and thought it would reach $100. We did the right thing to sell at $36. AKBA investors now seem to be patient and will give it a few more quarters to judge V uptake. So I am cautiously bullish at today’s PPS. Remember to be a contrarian. Don’t buy when I become ultra bullish. I am human just like the crowd.
I like ACHV best at this depressed price of $2.50. It is at multiyear lows, awaiting likely approval in less than 1 year. That’s another great reco from Chris, but it is dead money for the next 6 months or more. His only bomb was ACXP. I sold out of it recently to offset my big capital gain from TGTX, upon the announcement of a big reverse split. ACXP has gone down another 20% after the RS.
What a bomb ACXP was. Lost plenty on that.
Thanks for the great input. Did MM say when the launch in Europe was going to happen?
Hsainu analysis is next level. His $400 target after NDD approval in 2027-28 is, well, shocking, but I remember you coming up with a $90 number for the same results, so your current $16 target is a bit surprising. Given the current AKBA quote of $3.30/share those targets have a fantasyland feel but in reality they are not out of the question if one looks at TAM size. Perhaps you have a dose of newly found bearishness after the last ERs. Indeed, your criticism of the CEO is certainly not unfounded and the recent quarter seems to have put the kibosh in whatever momentum the stock had in June-July. If the stock is now in the doldrums it can also be attributed to the now 3+years old bear market that afflicts Pharma and Biotechs and those macro trends are certainly not caused by mgmt. Should inflation continue to abate and the Fed cut rates and switch to a less tight monetary policy, investors/capital may start looking again at these stocks in which case the continuing sector bear market may finally come to an end.
I had an emotional roller coaster today. I was unhappy about the intellectual masturbation from doing VOICE and VOCAL trials to confirm what are well established from INNOVATE and FOCUS. I felt that these trials which won’t finish for a year or more will be used as cautious excuses for Davita to procrastinate in launching V. But I cheered up when someone said that Davita is running their pilot trial to get acquainted with initial use of V before launching in a big way. The pilot won’t take long, and Davita will be ready to add many patients on V starting in Q4.
Also, someone said that a Big Pharma could sell V like hotcakes, but a small firm like AKBA will be slower. I replied that hsainu’s high revenue projections are plausible in the hands of BP. If TDAPA is extended for another 3 years at progressively lower rates, that might give AKBA enough time to achieve blockbuster sales in 5 years instead of the 2 years in the hands of BP. There is a huge difference between access and actual sales. It may take 5 years to have access converge with actuality.
NDD anemia approval for V in 2028 will multiply revenue several fold. I think NDD approval is likely.
PATIENCE, as you say.
This seems like very bad timing
Aug 8 (Reuters) – TG Therapeutics Inc (TGTX):
* TG THERAPEUTICS FILES FOR MIXED SHELF OFFERING, SIZE NOT DISCLOSED – FILING Source text: Further company coverage:
After a bit more digging I’m told this is a renewal of an existing shelf. Seems like they could offer a short press release explaining what they are doing.
It still doesn’t sound good. Mike Weiss is squandering money on his R&D fantasies. TGTX should have been a big cash cow which might have been $60-100 from that, or higher if bought out.
Chris, on TGTX, with whatever expertise you have in patent law, can you tell if Roche loses its US patent for IV Ocrevus in 2029 and EU patent in 2028, can a generic for IV Ocrevus enter the market after those dates? SubQ Ocrevus was approved Sept 2024, and their patent will probably run to about 2040. Does the new patent for subQ Ocrevus prevent generic competition for any form of Ocrevus? My wild guess is that the patent for subQ Ocrevus won’t prevent a generic IV form to enter. SubQ Ocrevus has a different composition and method from IV Ocrevus. I spoke socially to a patent attorney, but he handles electrical patents and said the patent rules for drugs could be totally different.
If generic IV Ocrevus enters the market, it will destroy the market for IV and coming subQ Briumvi. Insurance companies dictate which drug is approved for use, and generic anything is always preferred. If I am wrong about the patent rules, and IV or subQ Briumvi has long patent protection, and there is no generic available for a long time, TGTX is still a viable investment, but only much cheaper, probably way below $20.
MM, do you have knowledge about this?
Regretably, what I know abut Intellectual Property law would fit into a thimble and have room left over for the Chinese Army. I learned long ago to stay in my lane when it comes to the sundry specialties of legal practice.
Thanks for being honest. Most of what I know about anything is general principles. My best subjects in school were math, physics, physical chemistry. Biology and organic chemistry had some principles, but most of it was descriptive science. I wasn’t a good memorizer, so I was weaker in those subjects. Math is known as the queen of sciences, since everything can be derived from basic axioms. Whatever I didn’t remember on tests I could derive quickly at the time during the test. Same goes for classical physics.
I have remained a good speller. I use general principles of phonetics to spell prefixes and suffixes, putting words together. I loved the logic of grammar, with few broken rules. But English is not taught that way these days, according to what I read. Unfortunately, law has a few basic concepts, but most of them are arbitrary constructs that don’t apply to different branches of law. That’s why you can be expert in a few branches of law but know little about others. In primary care internal medicine, I know much of what cardiologists, GI, endocrinologists, urologists do, because there are basic principles common to all specialties.
Crypto. BTC $121,771. ETH now my top holding. $4271. I am up 107 percent. XLM -Stellar Lumens up 289 percent. $5k.
A bit off topic, but I see that ARKK picked up a good amount of The Trade Desk (TTD) after it dropped 60% on Friday. I’m not one for chasing stocks after a large drop, but… Does anybody have any insights?
Third party evaluation: Overall, TTD rated very bearish.
Earning excellent (earnings growth, earnings trend and earnings consistency, but projected P/E not good).
Financials very bearish (price to book, price to sales, free cash flow, although return on equity and LT debt to equity are both positive points).
Technicals are bearish (Poor relative strength to market, price strength, price strength and money flow out of the stock, but volume trend excellent)
Expert opinions overall very bearish (short interest, analyst ratings trend, industry relative strength, but insider activity positive)
I’ve been out of the country and not online so can someone, tell me if I should hold my CAPR shares or dump them now and take the loss, when is the next catalyst as I am not in favor of holding them for years in hopes of recovering, I would rather put that money to work elsewhere – appreciate everyone’s opinion.
The next real catalyst is FDA approval of deramiocel, but that won’t help you make a buy/sell decision on CAPR. Decide which scenario makes the most sense to you. One, FDA approval comes mid to late 2026 when the company is out of money. Sell now and take the loss which is probably not too much. Two, speculate on the behavior of hated Prasad who just returned to the FDA. Will he continue to be the one dimensional intellectual ass who requires phase 3 double blind placebo controlled studies for everything? If so, the likely excellent phase 3 HOPE 3 data will command approval, but the question is WHEN? Bureaucratic procedures could delay approval for 9 months, and then the company runs out of money. Three, if Prasad is a human being with compassion for DMD patients just as he should have compassion for his oncology patients with terrible cure rates, and he bends his intellectual orthodoxy in response to the community of DMD patients, parents and their doctors, and he is scared of threats that he has received already about the CRL, he will approve quickly. An accelerated approval (AA) will be welcomed by the community as well as investors. He may give approval in a few months from now. This was the position of Chris after the CRL. Prasad’s standing at the FDA and positive actions will award him the Nobel Peace Prize. There will be strings attached, such as post-marketing data on LVEF, clinical findings. That way, he will take pride in his supervision and think that if patients have some problems with the drug, he can gleefully malevolently withdraw it. Personally, I think this third scenario is most likely, so I am holding my large position at average cost of $8.52.
There are obvious risks. Personally, what I hate the most is selling at a loss, and missing the life-changing possibilities of holding until things play out. I think CAPR is well worth the gamble, and so is AKBA. My cost in AKBA is $1.75, so even if Vafseo uptake remains slow, and the stock goes back down to $2, I am happy to be patient for the more likely big gains in several years. I milked TGTX for what I thought it could do, so I felt comfortable by selling at $36 after holding for 5 or more years for 6X long term capital gain.
If you play it safe and sell, what will you do with the money? Even blue chips have their own risk/reward situation, and I don’t like spending hours trying to analyze things for minimal, although safer returns. There is an article about DECK from either Zacks or Simply Wall Street, saying that price/sales is higher for DECK than other apparel or shoe companies. DECK is worth a shot, but I missed the 5% upturn in the past few days. I think SPY is more reliable as a set it/forget it strategy. Fixed annuities are safe, low return vehicles, with a disadvantage that payouts are subject to highest ordinary tax rates. Stocks, for all their risks, have the highest long term returns, mainly because of favorable low capital gains tax rates. Just hold for 1 year to get low taxes.
I guess they are spending a lot for R&D and setting up distribution for Dur. What do you think of their vaccine technology?
There’s lots of excitement about CAPR’s StealthX platform for exosome development. NIAID is funding a phase 1 trial for vaccines. Exosomes can package anything you want for delivery. With the mRNA covid vaccine, I’ve always HATED the one-dimensional spike protein generated in high, prolonged exposure packaged in lipid nanoparticles which damage blood vessels. This is the antithesis of natural immunity achieved from exposure to the whole virus. Exosomes could package a smorgasbord of viral antigens and be a much closer mimic of natural immunity. The NIH is unhappy about mRNA vaccines which have faced backlash from the public. It is speculated that the exosome field could be worth $hundreds of billions.
If you want out sell the Sept calls. They will give you .65 cents for the 10 s and 30 cents for 12.50 s.
INO was out of the gate and leading the horse race today. One write up I read was it was on track to complete BLA for INO-3107 for RRP by end of 2025. Having requested rolling submission based on breakthrough therapy designation. JMP Securities maintained market outperform rating and a $12.00 price target. Today’s volume was 4,613,246. Hello MM. If they get FDA approval, what kind of a pop will result in the stock price? Is that $12.00 price target realistic? Thanks
New World Investor for 8.14.25 is posted. No issue next week. I’ll try to catch up on comments.