Dear New World Investor:
Last Friday’s Personal Consumption Expenditures Index was pretty much a nothingburger. Prices rose 2.5% year-over-year, the same as June despite President Trump’s broad-based tariffs. The month-over-month increase was 0.2%, down from 0.3% in June.
The year-over-year core PCE ticked up from +2.8% in June to +2.9% in July. Month-over-month core prices increased 0.3%, the same as June. The CME FedWatch Tool probability of a quarter-point cut in the Fed funds rate at the September 17 meeting actually ticked up a few points to 89.2%.
That was in spite of a 0.5% jump in consumer spending in July, the biggest increase since March. Spending jumped sharply for long-lasting goods such as cars, appliances, and furniture, many of which are imported. Incomes rose 0.4% from June to July, boosted by a healthy gain in wages and salaries.
But that won’t continue. Wednesday’s Job Openings and Labor Turnover Survey (JOLTS) report showed job openings, a measure of labor demand, dropped 176,000 to 7.181 million by July 31, below the consensus forecast for 7.378 million unfilled jobs.

Tomorrow’s August payrolls report has a very low consensus expectation of +75,000, probably due to the +73,000 shocker in July. Even if it comes in higher than this low bar, I still think the Fed cuts on September 17.
September often is a tough month for stocks. Since 1950, September has been the S&P 500’s weakest month of the year, posting an average decline of -0.7%, with positive returns only 44% of the time. Short term, Steve Deppe CMT pointed out that when the S&P 500 closes August at an all-time high, the Index has closed red for the month of September 14 of 18 times, for average losses of -1.60%. But, longer-term, the VIX Fear & Greed Index has fallen four months in a row. Historically, nine months later the S&P 500 was higher 100% of the time.

Market Outlook
The S&P 500 added only 0.22 points since last Thursday, but that was enough to squeeze out a new closing high today. The Index is still up 10.5% year-to-date. The Nasdaq Composite gained just 2.53 points, also to a new closing high today. It is up 12.4% for the year. The SPDR S&P Biotech Exchange-Traded Fund (XBI) climbed 3.5% as the new biotech bull rolled on. It is up 3.8% year-to-date. The small-cap Russell 2000 “soared” 1.4 points to a new 2025 high today and is up 6.7% in 2025.
The fractal dimension signaled a new thrust up, thanks to today’s strong recovery. Unless tomorrow’s payroll numbers seriously upset the apple cart, it looks like September might not be its usual downer.
Top 5
Changes this week: None
Near-Term – chronological order
AKBA Akebia Therapeutics – Vafseo launch
SCYX ScyNexis – Resolution of GSK situation
EQT EQT – natural gas price rebound
USL United States 12 Month Oil Fund, LP – crude should rise quickly
FCX Freeport McMoRan – copper shortage
Long-Term – alphabetical order
ABCL AbCelllera – Will become a huge pharma royalty company
UUUU Energy Focus – Domestic uranium supplier
EQT EQT – largest US natural gas company
IBIT iShares Bitcoin Trust – Bitcoin is headed for $150,000
META Meta – a (the?) leader in the metaverse
PLTR Palantir – a (the?) leader in AI applications software
SCYX ScyNexis –First new antifungal in 20 years
Economy
The Atlanta Fed’s GDPNow model ticked up to +3.0% as strength in both personal consumption expenditures growth and private domestic investment growth was partly offset by a sharp increase in imports, which reduce domestic GDP.
Coming Events
All times below are ET, and most presentations and slides are archived on the companies’ websites so you can listen to them.
Friday, September 5
INO – Inovio – 7:00am – H.C. Wainwright Global Investment Conference
August payrolls – 8:30am – +75,000; July was +73,000
Monday, September 8
DC – Dakota Gold – Through 9/10 – H.C. Wainwright Global Investment Conference
ABCL – AbCellera – 9:00am – Morgan Stanley Global Healthcare Conference
TGTX – TG Therapeutics – 10:30am – H.C. Wainwright Global Investment Conference
AKBA – Akebia – 12:00pm – H.C. Wainwright Global Investment Conference
NVDA – Nvidia – 12:25pm – Goldman Sachs Communacopia + Technology Conference
Tuesday, September 9
EDIT – Editas – Unspec. – Baird Global Healthcare Conference
DC – Dakota Gold – Through 9/12 – Precious Metals Summit, Beaver Creek
CMPS – Compass Pathways – 9:00am – H.C. Wainwright Global Investment Conference
Business Employment Dynamics – 10:00am – Annual payrolls revision; was -818,000 last year
CMPS – Compass Pathways (again!) – 10:45am – Morgan Stanley Global Healthcare Conference
GILD – Gilead Sciences – 11:00am – Morgan Stanley Global Healthcare Conference
AAPL – Apple – 1:00pm – iPhone 17 introduction
PYPL – PayPal – 4:45pm – Goldman Sachs Communacopia + Technology Conference
Wednesday, September 10
SCYX – ScyNexis – 10:30am – H.C. Wainwright Global Investment Conference
GILD – Gilead Sciences – 11:25am – Baird Global Healthcare Conference
Short Interest – After the close
FSLY – Fastly – 3:30pm – Piper Sandler Growth Frontiers Conference
Thursday, September 11
Consumer Price Index – 8:30am
Big Tech: The Biotech & Digital Dominators MegaShift
There are at least four ways to make money in the stocks of these large, growing, dominant companies. You can:
* * Buy a stock and hold it
* * Buy a stock and write a call option against it
* * With a Level IV options account, write an out-of-the-money put option
* * With a Level IV options account, write an out-of-the-money put option and use part of the premium to buy an out-of-the-money call option
Apple (AAPL – $239.78) will announce the iPhone 17 next Tuesday. They are planning to launch their own AI-powered web search tool next year called World Knowledge Answers that will be integrated into the Siri voice assistant. Apple is aiming to release the service next spring as part of a long-delayed overhaul to Siri.
The underlying technology enabling the new Siri probably comes at least in part from Google. The two companies reached a formal agreement this week for Apple to evaluate and test a Google-developed AI model to help power Siri.
MoffettNathanson upgraded Apple from Sell to Neutral with a $225 price target after getting their heads handed to them. They said a number of key headwinds have faded and the “worst-case scenarios are off the table.” That’s nice. AAPL is a Buy under $205.
Corning (GLW – $69.72) CFO Ed Schlesinger presented at the Citi Global Technology, Media, and Telecommunications Conference (AUDIO HERE and TRANSCRIPT HERE). It was the usual presentation these days, emphasizing how the AI buildout is keeping them on the internal and faster Springboard three-year growth plan.
China is imposing new anti-dumping duties on certain single-mode optical fibers originating from the U.S. starting today. The anti-dumping rate set on Corning is 37.9%. Don’t worry about it – China has placed such duties on U.S. optical fiber for more than a decade, with some rates as high as 78%.
UBS upgraded the stock from Neutral to Buy and raised their target price from $65 to $84. They wrote: “We upgrade GLW to Buy as we see ongoing AI-driven fiber growth continuing to exceed market expectations, driving sustainable higher growth and re-rating in the stock. We believe consensus is not modeling the full GLW fiber content uplift in AI datacenter as fiber density increases, outpacing hyperscaler capex growth in 2027-2029e. We expect Optical to deliver a ~27% sales CAGR through 2027 (2027e ~18% above consensus), driving much of our ~13% GLW sales CAGR over this period.”
They added that the total addressable market for fiber is continuing to expand, with data centers requiring between four and sixteen times more fiber content. Corning is signing contracts to connect datacenters inside their campuses and over long distances. In the longer-term, co-packaged optics will need another two to three times more fiber content, and with new innovations from Corning, companies can get between 40% and 80% more cabling in existing infrastructure.
Beyond that, UBS thinks there is a “sizable” opportunity in US solar, where Corning has roughly 80% of the restarted capacity over the next five years, which could boost annual sales by roughly $1.5 billion. GLW is a Hold for a $60 target in 2025.
Gilead Sciences (GILD – $112.77) is in a full-court press to get the stock re-rated up as an oncology play. Next week they are presenting at both the Morgan Stanley Annual Global Healthcare Conference and the Baird Global Healthcare Conference. This week it was Chief Medical Officer Dietmar Berger at the Cantor Global Healthcare Conference (AUDIO HERE and TRANSCRIPT HERE) and CFO Andy Dickinson at the Wells Fargo Healthcare Conference (AUDIO HERE and TRANSCRIPT HERE). The story is always the same: growth is back, oncology is working, the pipeline is deep, and – oh, did I mention? – growth is back.
The company broke ground on its new Pharmaceutical Development and Manufacturing Technical Development Center at their Foster City headquarters. The five-story, 180,000 ft2 facility will serve as a hub for innovation and collaboration across technical development and manufacturing teams. Designed with flexible pilot lab space and advanced digital infrastructure, it will accelerate technology transfer and support the advancement of next-generation biologics across Gilead’s pipeline. It will feature digitally enabled systems, autonomous robotics, and real-time digital monitoring, making it one of the most AI-enabled centers in the biopharma industry. GILD is a Long-Term Buy under $115 for a first target of $150.
Micron (MU – $124.21) is the obvious beneficiary as Washington revoked the authorizations of SK Hynix and Samsung Electronics to obtain U.S. semiconductor equipment for operations in China. The move is set to go into effect in 120 days.
The Commerce Department had granted the firms waivers from broad 2022 export controls on sales of American semiconductor equipment to China. The companies will now be required to secure licenses to purchase equipment for their China operations. According to the department, the US plans to approve licenses for the firms to keep operating their existing plants in China, but will not allow expansions or technology upgrades.
The South Korean chip makers control the bulk of global memory chip output, accounting for roughly 70% of the DRAM market—crucial for data centers and AI—and 54% of the NAND market. SK Hynix competes with Micron as a major supplier of high-bandwidth memory chips to Nvidia. Analysts estimate that 30% to 40% of SK Hynix’s DRAM and NAND output comes from China.
Samsung is expected to be less exposed, as only its NAND production is based there, although it’s still significant at about one-third of total production. That competes with Micron’s NAND products. MU is a Buy under $125 for a $200 target.
Nvidia (NVDA – $171.66) has a growing auto business that is poised for a big year. Their automotive segment revenues hit $586 million in the recently-reported second quarter, a 69% jump year-over-year. Management said the gain was primarily driven by its self-driving solutions.
CFO Colette Kress said they also began shipping their DRIVE AGX Thor SoC (system on chip), the successor to their Orin self-driving platform. On the earnings conference call, she said: “Thor’s arrival coincides with the industry’s accelerating shift to vision, language, model architecture, generative AI, and higher levels of autonomy. Thor is the most successful robotics and AV computer we’ve ever created. Thor will power our full stack DRIVE AV software platform – now in production – opening up billions in new revenue opportunities for Nvidia while improving vehicle safety and autonomy.”
Shareholders require a weekly YouTube from CEO Jensen Huang, and this one is a lulu:
NVDA is a Hold for a $180 first target.
Onsemi (ON – $48.06) CEO Hassane El-Khoury and CFO Thad Trent also presented at the Citi Global Technology, Media, and Telecommunications Conference (AUDIO HERE and TRANSCRIPT HERE). Hassane said they are seeing early signs – green shoots – of the turnaround in industrial and automotive markets.
I recognize that I am early in buying turnarounds like Corning, Micron, PayPal, and On (and Intel in Boomberg), and that is deliberate. It’s not possible to consistently pick the absolute bottom in either a company’s fortunes or its stock price, so the question is whether it is better to be early or late. I choose early because the change from “bad” to “less bad” often provides an outsize stock price move up that creates a comfortable cushion for further gains as the turnaround proceeds. Some people prefer to wait for the bottom and jump in after that first upswing confirms a bottom. If that’s you, great! Both approaches can work. I think we’ve seen that first bounce with ON.
ON is a Buy under $60 for a $100 first target.
Palantir (PLTR – $156.14) won another government sole source contract, this one from the State Department. They said: “Only Palantir and Foundry combine all the capabilities below into a single solution that can be setup, deployed, and managed in all use cases for State.”
They signed an important new contract with Lumen to bring Palantir’s Foundry and Artificial Intelligence Platform (AIP) to Lumen as it transforms its business from traditional telecom into a next-generation technology infrastructure company leveraging its physical fiber network, digitally powered platform, and connected ecosystem. Palantir’s Foundry and AIP will streamline workflows, accelerate decision-making, and simplify complex legacy operations, from customer service and compliance reporting to the heart of the transformation: Decommissioning legacy telecom infrastructure and migrating products into modernized ecosystems.
Lumen’s chief technology and product officer said: “As Lumen powers the backbone of the AI economy, we’re determined to make our own operations intelligent and efficient, just like the networks we deliver to our customers. Working with Palantir allows us to harness AI to accelerate our modernization efforts and deliver the network and services our customers need in the AI era.”
Ted Mabrey, Head of Palantir Commercial, said: “Lumen is redefining what’s possible in telecom by fusing AI into the very fabric of its operations. With Foundry and AIP, Lumen is accelerating its transformation into a technology infrastructure company. Their expansive network, digital platform, and connected ecosystem make them an ideal partner to showcase how AI can transform an industry at scale.”
Palantir also signed a five-year renewal with Lear (LEA), a global automotive leader in seating and E-Systems. Under the new agreement, Lear will broaden its use of Foundry, Palantir’s Warp Speed manufacturing operating system ,and Artificial Intelligence Platform (AIP), across its global manufacturing footprint. Currently, Foundry and AIP are helping Lear proactively manage its tariff exposure, automate administrative workflows, and dynamically balance its manufacturing lines. Palantir’s platforms connect functions across quality, supply chain, procurement, manufacturing, finance, and design, and today more than 11,000 Lear employees use Palantir’s technology to drive efficiency and innovation.
Palantir Foundry and AIP also have played an important role in Lear’s Innovative, Digital, Engineered and Automated (IDEA by Lear) program, which unifies Lear’s transformation initiatives. Lear’s implementation of IDEA has resulted in more than $30 million in savings during the first half of 2025.
Louis Mosely, head of Palantir UK, said: “We are at the very beginning of a technical revolution…of the scale of the Industrial Revolution.” Artificial Intelligence is not in a bubble and will revolutionize the world in “10 to 15 years.”
AIPCon 8 was held today with over 70 speakers from across Palantir’s US commercial customer base, including Waste Management, bp, MaineHealth, American Airlines, Lumen, TWG Motorsports, and Novartis. They discussed how they are using Palantir Foundry and AIP as the backbone for winning the nuclear power race against China, accelerating pharmaceutical R&D, managing complex responses to natural disasters, optimizing large-scale fleets, invigorating American manufacturing, delivering the right care to the right patient at the right time, and more.
PLTR is a Buy under $160 for a $200 first target.
PayPal Holdings (PYPL – $68.46) announced that Venmo and PayPal customers in the US and select global markets will be able to skip the wait-list and receive early access to Perplexity’s new AI-powered Comet browser. Comet offers a range of features like an integrated AI assistant, native answer-focused search, product comparisons, and more. I’ve been waiting for this! The new offer includes a free 12-month trial of Perplexity Pro, valued at $200.
CFO and COO Jamie Miller presented at the Jefferies Fintech Conference (VIDEO HERE and TRANSCRIPT HERE). She said the turnaround is on or even ahead of plan. PYPL is a Buy under $75 for a double in three years.
SoftBank (SFTBY – $52.94) said PayPay, their subsidiary, has filed a draft registration statement to go public in the US. SFTBY is a Buy under $35 for a first target of $50 and then higher as the discount to hard book value disappears.
Small Tech
Enovix (ENVX – $9.02) said that one day before the warrants expired, about 12.6 million had been exercised, bringing in $110.1 million. The warrants expired last Friday with an inherent value of 87¢ ($9.62 ENVX price minus $8.75 warrant exercise price). I’m going to subtract that from my original recommendation price of $12.64 to get an adjusted recommendation price of $11.77. ENVX is a Buy up to $20 for a 4-year hold to $100+ as their BrakeFlow lithium-ion battery takes market share.
Primary Risk: A new competitor invents a better battery.
PagerDuty (PD – $16.57) reported July second quarter revenues up 6.4% from last year to $123.41 million, in line with the consensus estimate. But pro forma earnings of 30¢ a share clobbered the 20¢ estimate, and they were GAAP profitable for the first time ever. On the conference call (VIDEO WITH TRANSCRIPT HERE and SLIDES HERE and TRANSCRIPT HERE), CEO Jennifer Tejada guided the September quarter to revenues of $124.0 to $126.0 million (4% to 6% year-over-year growth) with pro forma earnings of 24¢ to 25¢.
For the full year, she slightly reduced revenue guidance from $493-$499 million to $493-$497 million, but she increased pro forma earnings guidance from 95¢-$1.00 to $1.00-$1.04. They have made steady progress in the number of customers and revenue growth:
They appointed Todd McNabb as Chief Revenue Officer (CRO). He has more than 25 years of experience scaling companies across diverse industries, with a proven track record of driving growth within enterprise organizations. Most recently, he was Chief Revenue Officer at PROS, where he led global go-to-market operations. Prior to PROS, he was President and Chief Revenue Officer at ScienceLogic, where he delivered growth of 65% year-over-year in the first half of 2023 while transforming the company’s go-to-market strategy. PD is a Buy up to $30 for a 2- to 5-year hold as their digital operations management Software-As-A-Service gains market share.
Primary Risk: Digital operations management is a competitive area.
Biotech MegaShift
If you can afford it – and it would not be too big a position in your portfolio – putting $2,000 into each of these speculative biotechs might be a good way to start. Buying these out-of-favor, fallen, or forgotten companies that can get important products through the FDA at very low market capitalizations seems like a good strategy to me.
Risks
Development-stage biotechs are subject to investor sentiment swings from wildly optimistic to excessively pessimistic – mostly the latter recently. After the Primary Risk for each company, I’ve added the clinical stage of their lead product, the probable time of their first FDA approval, and the probable time of their next financing.
As always, you need to think about an appropriate position size. You could buy a full position upfront and then just hold on, or buy some upfront and leave room to add more on the inevitable financings, transient clinical trial setbacks, and the like.
Important: The FDA just introduced the Rare Disease Evidence Principles (RDEP) to provide greater speed and predictability in the review of therapies intended to treat rare diseases with very small patient populations with significant unmet medical need and that are driven by a known genetic defect. Through the RDEP process, sponsors will receive clearer guidance on the types of evidence that can be used to demonstrate substantial evidence of effectiveness.
It is well understood that developing drugs for rare diseases can make it difficult or even impossible to generate substantial evidence of safety and efficacy, as required by statute, using multiple traditional clinical trials. Instead, rare disease drug developers and the FDA can work together to identify alternative methods for meeting the statutory standard that are both rigorous and viable for rare disease populations.
This is a very welcome development, not only because it will make life easier for companies like Inovio, but because it encourages FDA employees who want to change the bureaucracy.
Akebia Therapeutics (AKBA- $3.02) CFO Erik Ostrowski and Chief Commercial Officer Nik Grund presented at the Wells Fargo Healthcare Conference (AUDIO AND SLIDES HERE) and they’ll repeat at Monday’s H.C. Wainwright Global Investment Conference.
They said the low-hanging fruit for Vafseo is home use – a pill instead of an IV – and those on high ESA doses.
DaVita, with over 2,800 clinics and 204,000 dialysis patients, has started their three-month operational pilot across more than 100 of their clinics. It is going very well, and after mid-November it will roll out to all of their clinics. Fresenius, with over 2,600 clinics and also with over 200,000 patients, will do their pilot next.
US Renal Care is the third-largest dialysis organization with over 400 clinics and about 36,000 patients. Over 80% of US Renal Care physicians have written a Vafseo prescription. Innovative Renal Care and Dialysis Clinic, Inc., the #4 and #5 clinics each with about 16,000 patients, came online in the third quarter.
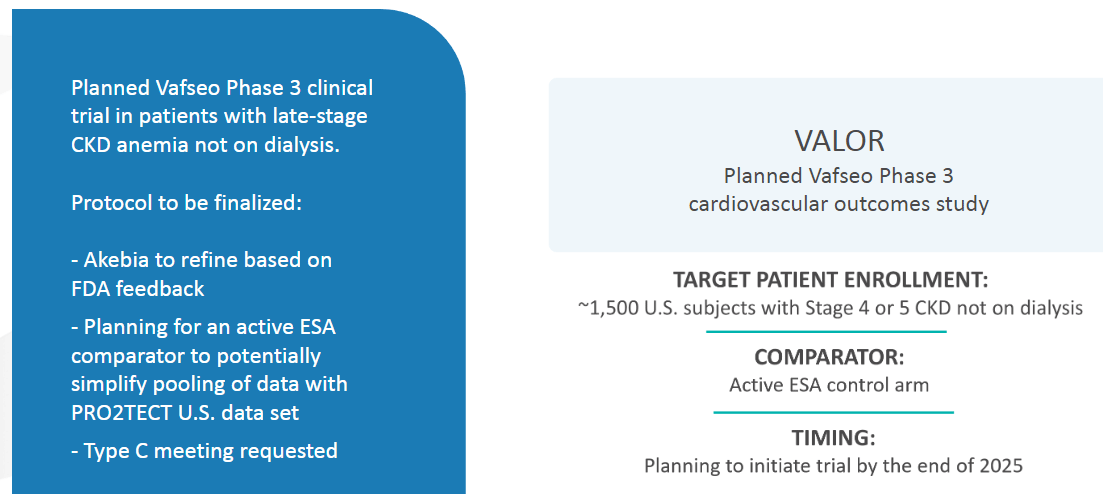
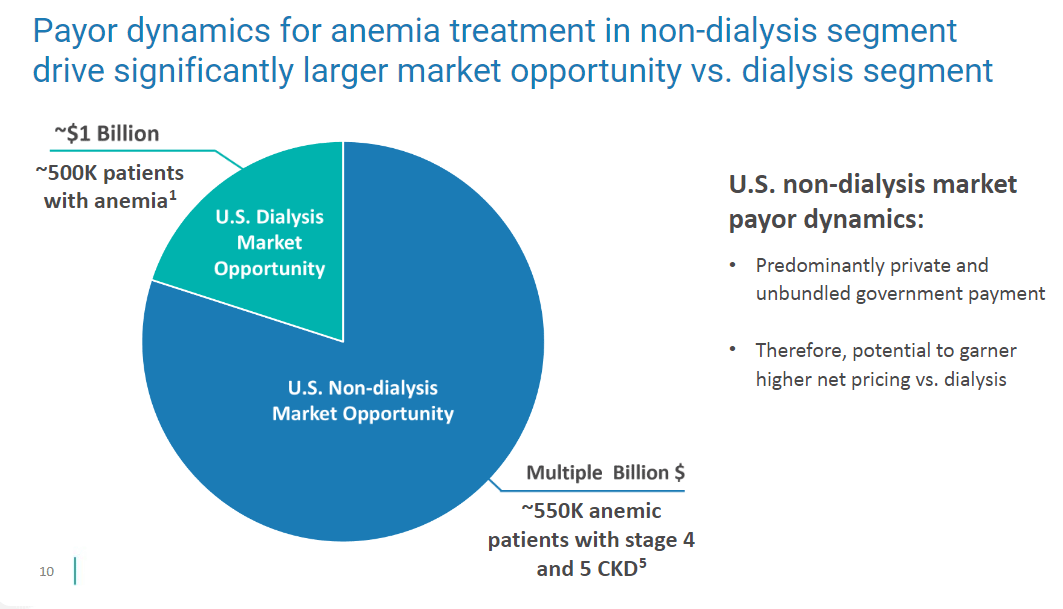
The next big opportunity is non-dialysis-dependent chronic kidney disease. They have the 1,500-patient trial designed and after an FDA Type C meeting that they have requested, they’ll start the trial by the end of the year.
They will charge a much higher price for Vafseo outside the dialysis bundle.
They are doing a clinical trial with U.S. Renal Care to show that Vafseo significantly lowers hospitalization rates compared to ESAs. It enrolled ahead of schedule in June. We’ll see data 18 months after the last patient was dosed, so early in 2027.
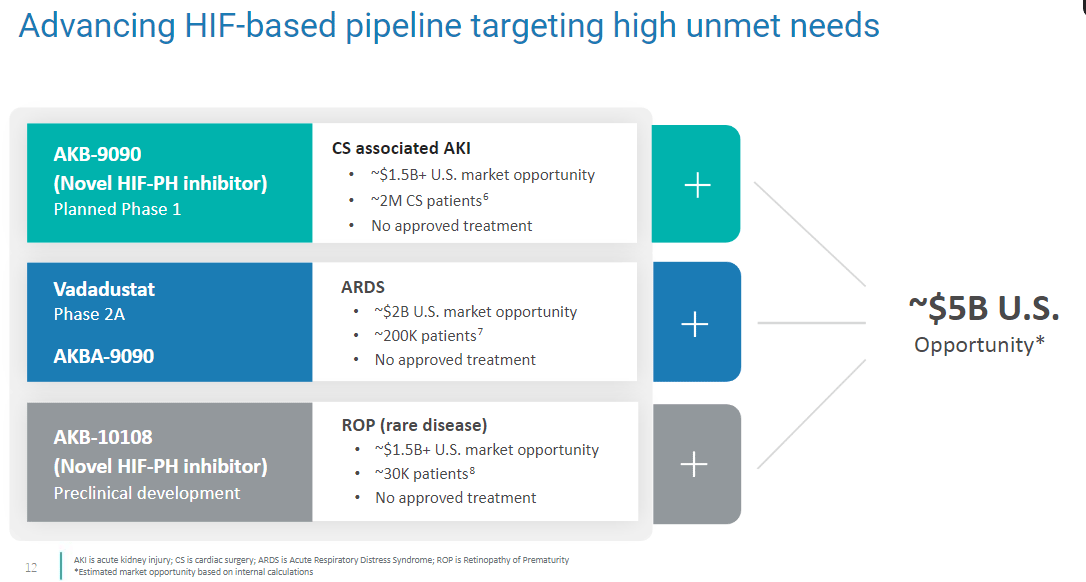
Akebia has a pipeline of new drugs that most investors don’t pay attention to, but would be very interesting to a Big Pharma acquirer. AKB-9090 should be in a Phase 1 trial before the end of 2025.
They said again that the company is funded to profitability. No equity sales needed. Buy AKBA up to $4 for the Vafseo launches in the EU, UK, and US. I think GSK and/or Amgen will make a bid for the company.
Primary Risk: Vafseo doesn’t sell in the US.
Clinical stage of lead product: Approved
Probable time of next approval: 2026
Probable time of next financing: Never
Compass Pathways (CMPS – $5.10) CEO Kabir Nath brought CMO Guy Goodwin and Chief Patient Officer Steve Levine to a fireside chat at the Cantor Global Healthcare Conference (AUDIO HERE and TRANSCRIPT HERE). With the Phase 3 trials underway, there’s not much new they can say. They continue to explain why they think their trial will meet the FDA’s placebo requirements, but at this point I think there are two types of people: those like me who believe they’ve nailed it and those who will only believe it when the FDA accepts it.
The company announced publication in the Journal of Psychopharmacology of the results from their open-label Phase 2 study of a single-dose of 25 milligrams of COMP360 in 22 patients with post-traumatic stress disorder (PTSD). COMP360 was well tolerated with both rapid and durable improvement in symptoms from baseline observed out to 12 weeks following a single administration. CMPS is a Buy under $10 for a very long-term hold to $200.
Primary Risk: Their drugs fail in the clinic.
Clinical stage of lead product: Phase 3
Probable time of first FDA approval: 2028
Probable time of next financing: Late 2025
Editas Medicine (EDIT – $2.52) announced the nomination of its lead in vivo development candidate, EDIT-401, an experimental, potential best-in-class, one-time therapy based on Editas’ differentiated upregulation approach designed to significantly reduce LDL cholesterol levels. It directly edits the LDLR gene to increase LDLR protein expression and reduce LDL-C levels. This targeted approach has demonstrated a favorable preclinical profile in both efficacy data and tolerability and supports the potential of EDIT-401 to deliver meaningful clinical outcomes for patients underserved by current lipid-lowering therapies.
On the webinar (AUDIO HERE and SLIDES HERE and TRANSCRIPT HERE), CEO Gilmore O’Neil and CSO Linda Burkly said they have seen robust efficacy data with about a 90% mean reduction of LDL-C in preclinical non-human primate studies, while standard of care therapies demonstrate a 40-60% mean reduction of LDL-C. Instead of taking statins for life, it is a one-time treatment designed for lifelong benefit. The potential market is huge.
The said they have compelling preclinical data that supports rapid progression to human proof-of-concept data by the end of 2026.
Gilmore presented at the Cantor Global Healthcare Conference (AUDIO HERE) and focused on EDIT-401 as the culmination of their pivot to a completely in vivo gene editing company. They have enough cash to get into the June 2027 quarter. EDIT is a Buy under $6 for a double in 12 months and a long-term hold to much higher prices.
Primary Risk: Other companies’ gene-sequencing drugs fail in the clinic.
Clinical stage of lead product: Partnered: Approved. Owned: Going into the clinic mid-2025.
Probable time of next FDA approval: 2028
Probable time of next financing: Late 2026 or never
Inovio (INO – $2.75) presents at the H.C. Wainwright Global Investment Conference tomorrow. INO is a Buy under $5 for a very long-term hold.
Primary Risk: Their drugs fail in the clinic.
Clinical stage of lead product: Phase 3
Probable time of first FDA approval: Mid-2026
Probable time of next financing:After FDA approval in 2026
ScyNexis (SCYX – $0.87) will have six presentations highlighting data from SCY-247, its second-generation fungerp, at the 12th Congress on Trends in Medical Mycology, September 19 through 22. They are doing an oral presentation featuring data demonstrating SCY-247 in vitro activity against candida auris strains, including isolates with mutations commonly associated with echinocandin-resistance. Additional poster presentations highlight SCY-247’s broad spectrum of antifungal activity against Candida species, including multidrug- and pandrug-resistant candida auris and aspergillus species.
The company will report top-line data from the Phase 1 trial of SCY-247 by the end of this month. Buy SCYX under $2.50 for a first target price of $20 after ibrexafungerp is approved for hospital use and a buyout at $50.
Primary Risk: Ibrexafungerp fails to sell.
Clinical stage of lead product: Approved
Probable time of next FDA approval: 2026
Probable time of next financing: Never
TG Therapeutics (TGTX – $31.89) completed its previously authorized $100 million stock buyback program, which was initially announced in August 2024. Under that program, they repurchased a total of approximately 3.5 million shares at an average price of $28.55 per share. The Board authorized a new program to acquire up to an additional $100 million of stock.
CEO Mike Weiss presented at the Cantor Global Healthcare Conference (AUDIO HERE and TRANSCRIPT HERE). As usual, Mike said the Briumvi launch is going well but didn’t provide any details. Buy TGTX under $30 for a target price in a buyout of $40 or more.
Primary Risk: Briumvi, the MS drug, fails to sell.
Clinical stage of lead product: Approved
Probable time of next FDA approval: NM
Probable time of next financing: Never
Inflation MegaShift
Gold ($3,596.20) shot past $3,500 an ounce to new record highs today at $3,602.00 intraday and $3,596.20 closing. Gold had a strong start in 2025, and by the third week of April was up 30%. But then came the inevitable consolidation, and it traded in a $3,200 to $3,400 range through the summer.
Now the next upleg has started, and finally – finally – gold miners, leveraged plays on the gold price, have been performing extremely well. The NYSE Arca Gold Miners Index was up more than 20% in August versus a 5.5% move in gold itself.
While the S&P 500 has delivered a more than respectable 40%+ return over the past two years, gold is up approximately 80%. After an extremely strong August, gold miners are now up more that 120%.

Next: The exchange-traded funds start chasing as retail piles in:

The fractal dimension signaled a new move up today – wait. Both the S&P 500 and gold bull market signals on the same day? It’s almost like someone has decided to print money and goose inflation. Looking at you, Jerome.
Miners & Related
Coeur Mining (CDE – $14.10) reported high-grade exploration results at both their Las Chispas underground silver and gold mine in Sonora, Mexico and the Kensington underground gold mine in Alaska. Las Chispas drilling hit bonanza grades in multiple veins. Drilling at the North Las Chispas vein, discovered in September 2024 and located immediately east of the existing underground infrastructure, returned exceptionally high-grade mineralization. Intercepts included 1.0 feet at 4.61 ounces per ton (oz/t) of gold and 392 oz/t of silver. Near-mine expansion and infill programs have been ramped up to nine drill rigs to target additional resource growth.
Kensington drilling delivered high grades and continuity in key veins, including multiple parallel veins returning high grades over mineable widths. One hole showed 7.1 feet at 11.5 oz/t. Quinton Hennigh, PhD, and Technical and Geological Advisor to Crescat Capital, said: “Nothing like seeing a major miner explore their high-grade deposits and proudly publish results. Need to see more of this. ”
CDE is a Buy under $10 for a $20 target as gold goes higher.
Primary Risk: Prices of precious metals fall due to US dollar strength.
Cryptocurrencies
Cryptocurrencies are a diversifying asset that offer a unique opportunity to make (or lose!) a lot of money quickly.
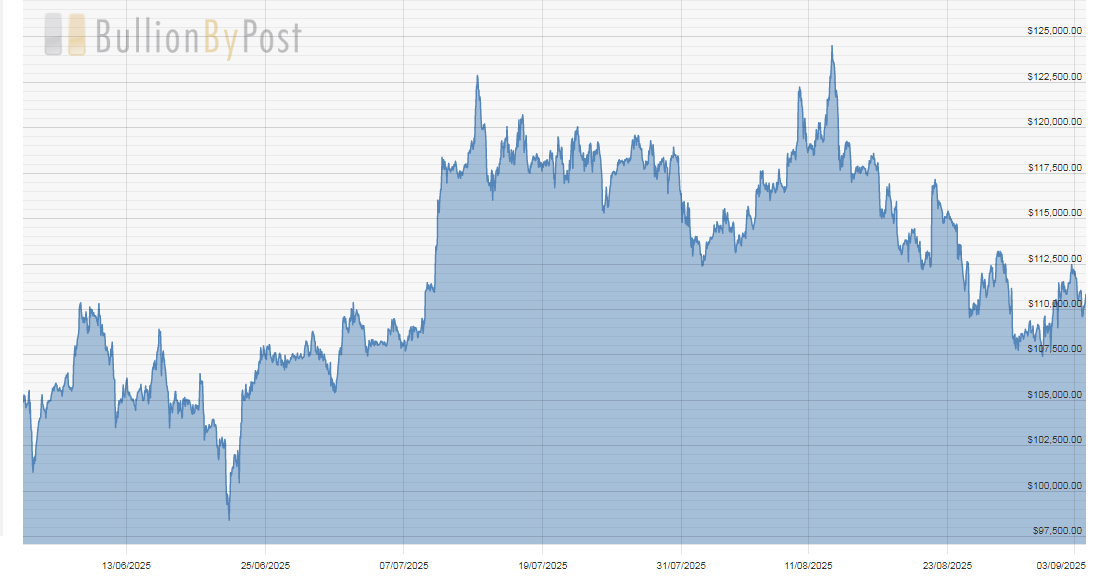
Bitcoin (BTC-USD on Yahoo – $110,813.03) is consolidating its run over $120,00 through a decline in price instead of the passage of time. Either way, the energy is building for the next uptrend that should top in the mid-$130,000s.
BTC-USD, ETH-USD, IBIT, and ETHA are Strong Buys.
Primary Risk: Bitcoin falls due to over-regulation or is surpassed by another cryptocurrency.
iShares Bitcoin Trust (IBIT- $62.35) remains the cheapest and easiest way to buy bitcoin. IBIT is a Buy for the 2028, 2032, and 2036 halvings.
Primary Risk:Bitcoin falls due to over-regulation or is surpassed by another cryptocurrency.
iShares Ethereum Trust (ETHA- $32.30) remains the cheapest and easiest way to buy ethereum. ETHA is a Buy for the coming explosion in token-funded start-ups.
Primary Risk: Ethereum falls due to over-regulation or is surpassed by another cryptocurrency.
Commodities
Oil – $63.34
Oil was hit after Reuters reported that OPEC+ will consider further raising output at a meeting on Sunday. That would mean starting to unwind the original cutbacks, which no one adheres to except Saudi Arabia. As I’ve been saying, always watch what they do – in this case, Saudi exports – and not what they say.

And how are President Trump’s sanctions on Russian and Iranian crude exports doing? About as I expected. Russian exports are up 532,000 barrels a day from this time last year. Iran’s are up 100,000 barrels a day.
The July 2026 Crude Oil Futures (CLN26.NYM – no trades – June closed at $61.97) are a Buy under $70 for a $200+ target. Only buy futures for all cash; do not use margin.
The United States 12 Month Oil Fund, LP (USL – $35.87) is a Buy under $40 for a $100+ target.
Vermilion Energy (VET – $7.49) is a Buy under $11 for a target price of $24 or more.
Primary Risk: Oil prices fall.
EQT (EQT – $51.80) will rise as natural gas prices rise going into winter. European Union natural gas inventories are 77.65% full, just below the five-year average. In declining order, Italy is at 89.14%; France is at 86.53%; Spain at 85.32%; Austria at 81.28%; Germany at 71.05%; Netherlands at 64.80%.

EQT is a buy under $70 for a long-term hold for much higher prices.
Primary Risk:Natural gas prices fall.
* * * * *
Suggested by subscriber StephKam
* * * * *
Your understanding why robots lag behind AI chatbots Editor,
Michael Murphy CFA
Founding Editor
New World Investor
All Recommendations
Priced 09/04/25. Check out the complete Portfolio page HERE.
Buys
These are the stocks everyone needs to own because transformative events are happening over the next year or two, and I expect to hold them long-term.
Tech Dominators
Apple Computer (AAPL – $239.78) – Buy under $205
Gilead Sciences (GILD – $112.77) – Buy under $115, first target price $150
Meta (META – $748.65) – Buy under $705 for a long-term hold
Micron Technology (MU – $124.21) – Buy under $125, target price $200
Onsemi (ON – $48.06) – Buy under $60, first target price $100
Palantir (PLTR – $156.14) – Buy under $160 for $200 first target price
PayPal (PYPL – $68.46) – Buy under $75, target price $150
Snap (SNAP – $7.09) – Buy under $11, target price $17+
SoftBank (SFTBY – $52.94) – Buy under $35, target price $50+
Small Tech
Enovix (ENVX – $9.02) – Buy under $20; 4-year hold to $100+
First Trust NASDAQ Cybersecurity ETF (CIBR – $72.35) – Buy under $75; 3- to 5-year hold
Fastly (FSLY – $7.30) – Buy under $10 for a 3- to 5-year hold to $50+
PagerDuty (PD – $16.57) – Buy under $30; 2- to 5-year hold
QuickLogic (QUIK – $5.12) – Buy under $10, target price $40
ARK Venture Fund (ARKVX – $35.89) – Buy for SpaceX
$20-for-$1 Biotech
AbCellera Biologics (ABCL – $4.16) – Buy under $6, target $30+
Akebia Biotherapeutics (AKBA – $3.02) – Buy under $4, target $20
Compass Pathways (CMPS – $5.10) – Buy under $10, hold a long time for a 20x return
Editas Medicines (EDIT – $2.52) – Buy under $6 for a double in 12 months and a long-term hold to much higher prices
Inovio (INO – $2.75) – Buy under $5, hold a long time
Medicenna (MDNAF – $0.78) – Buy under $3, first target $20, then maybe $40
ScyNexis (SCYX – $0.87) – Buy under $2.50, target price $20, then $50
TG Therapeutics (TGTX – $31.89) – Buy under $30 for buyout at $40+
Inflation
A Short-Sale or REO House – ($415,400) – Hold
Bag of Junk Silver – ($40.80) – hold through silver bull market
Sprott Gold Miners ETF (SGDM – $54.37) – Buy under $50, target price $75
Sprott Junior Gold Miners ETF (SGDJ – $59.56) – Buy under $60, target price $100
Sprott Physical Gold and Silver Trust (CEF – $33.08) – Buy under $35, target price $60
Global X Silver Miners ETF (SIL – $60.27) – Buy under $60, target price $100
Coeur Mining (CDE – $14.10) – Buy under $10, target price $20
First Majestic Mining (AG – $9.07) – Buy under $11, next target price $23
Paramount Gold Nevada (PZG – $1.04) – Buy under $1, first target price $10
Cryptocurrencies
Bitcoin (BTC-USD – $110,813.03) – Buy
iShares Bitcoin Trust (IBIT – $62.35) – Buy
Ethereum (ETH-USD – $4,323.83)– Buy
iShares Ethereum Trust (ETHA- $32.30) – Buy
Commodities
Crude Oil Futures – July 2026 (CLN26.NYM – no trades; June closed at $61.97) – Buy under $70; $200+ target
United States 12 Month Oil Fund, LP (USL – $35.87) – Buy under $40; $100+ target
Vermilion Energy (VET – $7.49) – Buy under $11; $24+ target
Energy Fuels (UUUU – $11.23) – Buy under $8; $30 target
EQT (EQT – $51.80) – Buy under $70; hold for much higher prices ($100+)
Freeport McMoRan (FCX – $46.07) – Buy under $50; $70 target within two years
Holds
These are holds but not sells – yet. They could get moved back to one of the buy categories if their prices drop or outlook improves, or they could become sell recommendations in the future.
Corning (GLW – $69.72) – Hold for $70
Nvidia (NVDA – $171.66) – Hold for $180 first target price
Dakota Gold (DC – $4.35) – Hold for $6 target price
Sandstorm Gold (SAND – $11.22) – Hold for Royal Gold acquisition
Publisher: GwynRose LLC, 5348 Vegas Drive, Suite 868, Las Vegas, NV 89108
New World Investor does not act as a personal investment adviser or advocate the purchase or sale of any security or investment for any specific individual. The recommendations and analysis presented to members are for the exclusive use of members. Members should be aware that investment markets have inherent risks and there can be no guarantee of future profits. Likewise, past performance does not assure future results. Recommendations are subject to change at any time. Nothing in this presentation should be considered personalized investment advice. No communication to you by Michael Murphy or any of our employees or contractors should be deemed as personalized investment advice.
Copyright ©GwynRoseLLC 2025
New World Investor Mastermind Group
1. Post unto others as you would have them post unto you.
2. Keep it clean, like a 1950s family television show. Your alter ego can run free on Twitter.
3. NO PERSONAL ATTACKS! If you don’t like the stock, don’t trash the person. Everyone is responsible for their own due diligence and investments.
4. Don’t post here about politics or religion – you aren’t going to change anyone’s mind. Again, NO PERSONAL ATTACKS!
5. The investment implications of something going on in politics or religion is OK.
6. Of course, there’s never a reason to slur someone based on race, religion, gender, sexual orientation, or country of national origin.
7. Please, no snark!
 Print This Post
Print This Post












Corning is a quarter away from your target of $70. Are you raising it to $84? Are we selling at $70? Or? Also when does Vafseo launch in the EU, UK and US? Finally, INO is on the move. What does INO have in the pipeline besides the product that looks like a FDA done deal soon?
MM…again ask why the source of fractals charts for S&P and gold are not identified and why you do not add and cover silver which may likely out perform gold going forward. All other charts are attributed. This raises questions about the veracity of your fractals charts in my mind and apparent desire to keep subscribers from knowing your sources.
Medicenna to Participate at the H.C. Wainwright 27th Annual Global Investment Conference – Medicenna Therapeutics
I see Dan Ives has launched a tech / AI ETF, anyone have any thoughts on that and it’s make up? Dan has been mentioned many many times by Mike here over the years and seems to have his finger on the pulse most of the time.
I’m not a big fan of ETF’s in general, but it covers a lot of the tech companies I’m interested in and don’t have the cash to invest in all of them individually !
My equivalent retail index fund is called Berkshire !
In Case You Missed It —
FDA TAPS BIOTECH VETERAN DR. NICOLE VERDUN AS NEW CDER DIRECTOR: A STRATEGIC SHIFT FOR DRUG APPROVAL AND INNOVATION
Washington D.C. – In a significant move signaling a potential recalibration of its approach to drug regulation and innovation, the U.S. Food and Drug Administration (FDA) has announced the appointment of Dr. Nicole Verdun as the new Director of the Center for Drug Evaluation and Research (CDER). Dr. Verdun, a seasoned professional with extensive experience in the biopharmaceutical industry, is poised to lead one of the FDA’s most critical centers, responsible for evaluating and approving approximately 20% of all products reaching American consumers.
This appointment marks a departure from the previous leadership, bringing a deep understanding of the complexities and rapid advancements within the biotechnology sector directly into the heart of drug review. The FDA’s decision to select a “biotech vet” underscores a growing emphasis on fostering innovation while maintaining rigorous safety and efficacy standards for new medicines.
More … https://www.asgct.org/publications/news/august-2023/policy-summit-nicole-verdun-director-fda-cber-otp
Of course, 8 weeks ago there was this:
Nicole Verdun, MD, director of the Office of Therapeutic Products (OTP) within the Food and Drug Administration’s Center for Biologics Evaluation and Research (CBER), has been placed on administrative leave, according to a report by STAT News. Rachael Anatol, PhD, deputy director of OTP, was also placed on leave.
Verdun joined FDA in 2012 and previously led the Office of Blood Research and Review (OBRR) before becoming director of OTP upon its establishment in 2023. The new office was created through a reorganization of the former Office of Tissues and Advanced Therapies to address the increasing complexity and volume of cell and gene therapy (CGT) products. More … https://www.aabb.org/news-resources/news/article/2025/06/20/regulatory-update–fda-s-nicole-verdun-placed-on-administrative-leave
The Biden Admin may have been a mess, but at least it was consistent. Mercurial Trump makes planning difficult.
This news about Verdun is positive for CAPR, although the bureaucracy may not permit Verdun with CDER to have influence on CBER people who determine the fate of CAPR.
The 1st linked article is from Aug 2023. I guess the news about CDER is true.
SAN DIEGO, Sept. 09, 2025 (GLOBE NEWSWIRE) — Capricor Therapeutics (CAPR) , a biotechnology company developing transformative cell and exosome-based therapeutics for rare diseases, today issued a statement regarding the U.S. Food and Drug Administration’s (FDA) public posting of its Complete Response Letter (CRL) for the Biologics License Application (BLA) for Deramiocel, the Company’s investigational cell therapy for the treatment of cardiomyopathy associated with Duchenne muscular dystrophy (DMD).
The Company was not notified in advance that the CRL would be posted, but acknowledges the FDA’s decision to publish the letter, originally received in July 2025. However, the FDA did not release the comprehensive preliminary response that Capricor submitted shortly after receipt of the CRL. This written response provided clarifications to the Agency’s feedback and outlined the Company’s proposed plan to address the outstanding issues. To ensure transparency, Capricor will make its preliminary response available on the investor section of its website for patients, families, and other stakeholders to review.
“Transparency is vital in regulatory communications, especially when patients are waiting for therapies with the potential to alter the course of devastating diseases such as Duchenne muscular dystrophy,” said Linda Marbán, Ph.D., Chief Executive Officer. “Our focus remains on working closely with the FDA to resolve the outstanding issues and to advance Deramiocel toward approval. While we respect the FDA’s process, we believe it is important that the public has visibility into both the CRL and our detailed written response submitted to the Agency. We are now awaiting the official minutes from our recent Type A meeting with the FDA review team, expected to be issued later this quarter, which will help define the next steps in our regulatory pathway. Looking ahead, we expect topline HOPE-3 data in the fourth quarter of 2025, and our discussions with the FDA have centered on how these data will inform and support the timing of our BLA resubmission.”
More impressive is Linda’s rebuttal of the CRL which was written by stupid AI. As an academic lab researcher for decades, she knows statistics better than out of context AI generated snippets from the CRL. I am not knowledgeable about statistics, so I can’t judge for myself, but I trust the CAPR statisticians in their rebuttal more than those at the FDA.
I see that people panicked last night when CAPR mentioned the shelf offering. Is it possible they got word back from the FDA and they want to be prepared for production?
Anybody still in this? Is this dead money for the next 10 months??
ACHV is my most confident bet right now. I bought more months ago after the big dilutive capital raise. Nice rebound since then. 9.5 months to wait is much better than worrying whether most spec NWI investments will break even before you die.
I understand your comment about NWI investments, but are you concerned about this dropping under 2 again with no catalysts untill next June?
Douglas, why worry that it might drop under $2? You obviously believe (as I do) that a big catalyst is 10 months away and that at that point the price will be much higher. If it drops under $2 in the meanwhile, that will be an opportunity to add shares or Call options, but certainly not to sell. If we see the price rise as ACHV approaches its pdufa date, you will be happy you already got in. It could very well be dead money for the next 10 months, but if you expect triple digit returns from here, why worry about the possibility of a lower price between now and then?
How about PPS in triple digits after a few years? With ARNA’s obesity drug, the PPS plunged after approval, so seasoned investors correctly sold on approval. Currently, wt loss drugs like GLP-1 agonists are much more effective, although with lots of risks. For antismoking drugs, ACHV’s will be the most effective. The question is whether insurers will cooperate and draw in the smoking and vaping population. If nothing else, vaping approval will come later and provide another boost to the stock after approval for smoking cigarettes only.
The Affordable Care Act requires insurers to pay for tobacco cessation treatments. 🙂
That would be great, but it is up to ACHV to do marketing to draw in smokers and vapers. I still see people spending money on nicotine gums and patches. This reminds me of those scam products to boost testosterone. I tried a few of them years ago, but nothing works except testo itself. Self injected IM testo costs only $100 cash yearly. I refuse to fill out prior authorization forms so patients can get insurance to pay. Nobody pays for my time to do PA forms.
ACHV can print money if they market properly. If so, target is over $100 in a few years.
The main risk of going back to 2 is from shorts right before approval. Shorts want to accumulate more shares at cheaper prices. Again I repeat my advice not to look for catalysts. Everyone knows what will move the stock–pdufa on June 20. When catalysts are known, the market price reflects that. Impatient retail investors usually lose. Often the stock moves up a few months in advance of pdufa. Don’t be left with no position.
With AKBA, the stock sold off after the brief pdufa bump. When Butler announced the 9 month launch delay until TDAPA could be implemented, I accumulated most of my big position during that time while waiting the 9 months for launch.
Hopefully we’ll have more days like this!!
I hope Chris and I convinced you to hold, even buy more below $2.50. There’s plenty of money to be made just holding the stock. I don’t want to play games with options which have time risk. There’s always time risk with the crap political FDA, as we are seeing with CAPR. CAPR may be a big winner, but I withdraw my comment of a few weeks ago that I have the most comfort with CAPR. ACHV has the advantage of the Affordable Care Act. AKBA has the advantage of TDAPA.
My modest valuation of ACHV. The latest Sept 2025 presentation on their site (they don’t have the replay of the HCW conference 9/8)–$11 billion market opportunity. Chantix peaked at 2.8 million scripts, or $800 million annual sales. ACHV’s drug candidate is a better version of Chantix, much lower % of side effects, although not zero. Conservatively, I’ll say $1 billion for ACHV’s drug, only 9% uptake. For peak revenue of $1 billion, use price/sales of 5 for $5 billion market cap (MC) at peak sales in several years. Current PPS is $3.17 at MC of $162 million. That’s $19.57 at $1 billion MC, or almost $100 PPS at $5 billion MC. This assumes no share dilution. How long will it take for ACHV to break even and then not require dilution? Cash runs out 2H 2026. There will be expenses for the vaping trial, July 2026 launch and continued marketing. In 2030, after 4 years of marketing, both cigarettes and vaping will have great sales. If the drug has $2 billion in sales, still well below $11 billion potential, the diluted stock could be $100-200 in 5-10 years. If you’re over 80 today, sell on approval at maybe $15. I’m 72 and will probably hold for much higher prices.
Does this help or hurt ACHV??
https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-fast-track-nicotine-pouch-reviews-amid-white-house-pressure-2025-09-09/
These Zyn common nicotine patches have lots of side effects. ACHV’s concept is far more innovative. I didn’t see any clinical trials for the Zyn ultra patch. The sales pitch is more flavors and higher doses. Good luck with higher doses–more addiction to nicotine. More flavors reminds me of kids getting addicted to vaping by being enticed by cool flavors. Immoral and stupid marketing.
After ACHV’s drug gets approved, they can go after the scumbag marketing for Zyn ultra products.
I agree. They should be fast tracking ACHV’S review instead of this. Unfortunately, your going against big companies like Altria and Phillip Morris who have deep pockets!!
Enovix: Utter repulsive, abusive, stock promoters at it again. Never a comment about customer orders or production volumes, backlog or deliveries. Now a convert with derivative transactions obfuscating the realities of the instrument. Dont forget the CEO unloaded shares at $15 during the warrant dividend brief honeymoon. True dirt bags IMO.
https://ir.enovix.com/node/12481/pdf
I’ve stayed away because of lack of transparency about customer orders, production volumes, backlog, deliveries. Scams and lies?
MM?
IREN and CIFR were both up over 30% the last two days.
Is MM AWOL? Ok? I don’t see any comments on anyone’s post??
New World Investor for 9.11.25 is posted.